Choline promotes nicotinic receptor alpha4 + beta2 up-regulation
- PMID: 20392695
- PMCID: PMC2888390
- DOI: 10.1074/jbc.M110.108803
Choline promotes nicotinic receptor alpha4 + beta2 up-regulation
Abstract
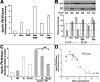
Neuronal nicotinic acetylcholine receptors (nAChR) composed of alpha4 + beta2 subunits, the high affinity nicotine-binding site in the mammalian brain, up-regulate in response to chronic nicotine exposure. The identities of endogenous mediators of this process are unknown. We find that choline also up-regulates alpha4 + beta2 nAChRs stably expressed by HEK293 cells as measured by increased [(3)H]epibatidine density. Choline-mediated up-regulation is dose-dependent and corresponds with an increase in beta2 subunit protein expression. The choline kinase inhibitor hemicholinium-3 inhibits approximately 60% of choline-mediated up-regulation revealing both an HC3-dependent and -independent pathway. Furthermore, choline-mediated up-regulation is not additive with up-regulation agents such as nicotine, but it is additive with weaker promoters of the up-regulation process. When co-applied with the pro-inflammatory cytokine tumor necrosis factor alpha, choline-mediated up-regulation is increased further through a mechanism that includes an increase in both alpha4 and beta2 protein expression, and this is inhibited by the p38 MAPK inhibitor SB202190. These findings extend the view that up-regulation of alpha4 + beta2 nAChRs is a normal physiological response to altered metabolic and inflammatory conditions.
Figures
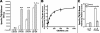


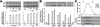
Similar articles
-
Upregulation of Nicotinic Acetylcholine Receptor alph4+beta2 through a Ligand-Independent PI3Kbeta Mechanism That Is Enhanced by TNFalpha and the Jak2/p38Mapk Pathways.PLoS One. 2015 Nov 30;10(11):e0143319. doi: 10.1371/journal.pone.0143319. eCollection 2015. PLoS One. 2015. PMID: 26619345 Free PMC article.
-
Reduced α4 subunit expression in α4+- and α4+- /β2+- nicotinic acetylcholine receptors alters α4β2 subtype up-regulation following chronic nicotine treatment.Br J Pharmacol. 2018 Jun;175(11):1944-1956. doi: 10.1111/bph.13896. Epub 2017 Aug 1. Br J Pharmacol. 2018. PMID: 28585241 Free PMC article.
-
Tumor necrosis factor alpha enhances nicotinic receptor up-regulation via a p38MAPK-dependent pathway.J Biol Chem. 2008 Jan 11;283(2):693-9. doi: 10.1074/jbc.M707330200. Epub 2007 Oct 31. J Biol Chem. 2008. PMID: 17977823
-
Engineered α4β2 nicotinic acetylcholine receptors as models for measuring agonist binding and effect at the orthosteric low-affinity α4-α4 interface.Neuropharmacology. 2015 May;92:135-45. doi: 10.1016/j.neuropharm.2014.12.035. Epub 2015 Jan 13. Neuropharmacology. 2015. PMID: 25595102
-
Nicotinic receptor subunit alpha5 modifies assembly, up-regulation, and response to pro-inflammatory cytokines.J Biol Chem. 2010 Aug 20;285(34):26049-57. doi: 10.1074/jbc.M110.105346. Epub 2010 Jun 21. J Biol Chem. 2010. PMID: 20566638 Free PMC article.
Cited by
-
Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain.J Pharmacol Exp Ther. 2012 Nov;343(2):441-50. doi: 10.1124/jpet.112.198085. Epub 2012 Aug 16. J Pharmacol Exp Ther. 2012. PMID: 22899752 Free PMC article.
-
Systematic characterization of cell type-specific master metabolic regulators in Alzheimer's disease.Res Sq [Preprint]. 2025 Aug 18:rs.3.rs-7207381. doi: 10.21203/rs.3.rs-7207381/v1. Res Sq. 2025. PMID: 40894034 Free PMC article. Preprint.
-
Nicotinic receptor alpha7 expression identifies a novel hematopoietic progenitor lineage.PLoS One. 2013;8(3):e57481. doi: 10.1371/journal.pone.0057481. Epub 2013 Mar 1. PLoS One. 2013. PMID: 23469197 Free PMC article.
-
Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons.J Neurochem. 2011 Oct;119(1):153-64. doi: 10.1111/j.1471-4159.2011.07408.x. Epub 2011 Sep 1. J Neurochem. 2011. PMID: 21806615 Free PMC article.
-
Endogenously expressed muscarinic receptors in HEK293 cells augment up-regulation of stably expressed α4β2 nicotinic receptors.J Biol Chem. 2011 Nov 18;286(46):39726-37. doi: 10.1074/jbc.M111.289546. Epub 2011 Sep 22. J Biol Chem. 2011. PMID: 21940627 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

