Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos
- PMID: 20392742
- PMCID: PMC2860247
- DOI: 10.1242/dev.046797
Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos
Abstract
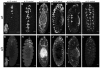
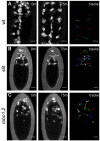
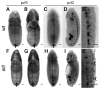
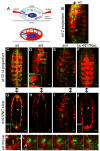
During embryonic development, Drosophila macrophages (haemocytes) undergo a series of stereotypical migrations to disperse throughout the embryo. One major migratory route is along the ventral nerve cord (VNC), where haemocytes are required for the correct development of this tissue. We show, for the first time, that a reciprocal relationship exists between haemocytes and the VNC and that defects in nerve cord development prevent haemocyte migration along this structure. Using live imaging, we demonstrate that the axonal guidance cue Slit and its receptor Robo are both required for haemocyte migration, but signalling is not autonomously required in haemocytes. We show that the failure of haemocyte migration along the VNC in slit mutants is not due to a lack of chemotactic signals within this structure, but rather to a failure in its detachment from the overlying epithelium, creating a physical barrier to haemocyte migration. This block of haemocyte migration in turn disrupts the formation of the dorsoventral channels within the VNC, further highlighting the importance of haemocyte migration for correct neural development. This study illustrates the important role played by the three-dimensional environment in directing cell migration in vivo and reveals an intriguing interplay between the developing nervous system and the blood cells within the fly, demonstrating that their development is both closely coupled and interdependent.
Figures




References
-
- Battye R., Stevens A., Jacobs J. R. (1999). Axon repulsion from the midline of the Drosophila CNS requires slit function. Development 126, 2475-2481 - PubMed
-
- Bruckner K., Kockel L., Duchek P., Luque C. M., Rorth P., Perrimon N. (2004). The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 7, 73-84 - PubMed
-
- Carmeliet P., Tessier-Lavigne M. (2005). Common mechanisms of nerve and blood vessel wiring. Nature 436, 193-200 - PubMed
-
- Cho N. K., Keyes L., Johnson E., Heller J., Ryner L., Karim F., Krasnow M. A. (2002). Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108, 865-876 - PubMed
-
- Choi K., Kennedy M., Kazarov A., Papadimitriou J. C., Keller G. (1998). A common precursor for hematopoietic and endothelial cells. Development 125, 725-732 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

