Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases?
- PMID: 20392809
- PMCID: PMC2879913
- DOI: 10.1124/pr.109.002469
Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases?
Abstract
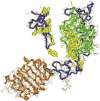
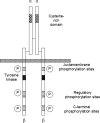
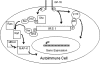
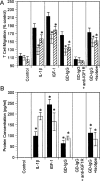
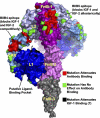
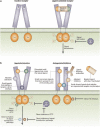
This topically limited review explores the relationship between the immune system and insulin-like growth factors (IGF-I and IGF-II) and the proteins through which they act, including IGF-I receptor (IGF-IR) and the IGF-I binding proteins. The IGF/IGF-IR pathway plays important and diverse roles in tissue development and function. It regulates cell cycle progression, apoptosis, and the translation of proteins. Many of the consequences ascribed to IGF-IR activation result from its association with several accessory proteins that are either identical or closely related to those involved in insulin receptor signaling. Relatively recent awareness that IGF-I and IGF-IR regulate immune function has cast this pathway in an unexpected light; it may represent an important switch governing the quality and amplitude of immune responses. IGF-I/IGF-IR signaling may also participate in the pathogenesis of autoimmune diseases, although its relationship with these processes seems complex and relatively unexplored. On the one hand, IGF-I seems to protect experimental animals from developing insulin-deficient diabetes mellitus. In contrast, activating antibodies directed at IGF-IR have been detected in patients with Graves' disease, where the receptor is overexpressed by multiple cell types. The frequency of IGF-IR+ B and T cells is substantially increased in patients with that disease. Potential involvement of IGF-I and IGF-IR in the pathogenesis of autoimmune diseases suggests that this pathway might constitute an attractive therapeutic target. IGF-IR has been targeted in efforts directed toward drug development for cancer, employing both small-molecule and monoclonal antibody approaches. These have been generally well-tolerated. Recognizing the broader role of IGF-IR in regulating both normal and pathological immune responses may offer important opportunities for therapeutic intervention in several allied diseases that have proven particularly difficult to treat.
Figures





References
-
- Abroun S, Ishikawa H, Tsuyama N, Liu S, Li FJ, Otsuyama K, Zheng X, Obata M, Kawano MM. (2004) Receptor synergy of interleukin-6 (IL-6) and insulin-like growth factor-I in myeloma cells that highly express IL-6 receptor α [published erratum appears in Blood 103:2891, 2004]. Blood 103:2291–2298 - PubMed
-
- Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. (2007) Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 179:3351–3361 - PubMed
-
- Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, et al. (2003) Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 75:1977–1983 - PubMed
-
- Ambrosini G, Adida C, Altieri DC. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

