Quantitative analysis of processive RNA degradation by the archaeal RNA exosome
- PMID: 20392821
- PMCID: PMC2926604
- DOI: 10.1093/nar/gkq238
Quantitative analysis of processive RNA degradation by the archaeal RNA exosome
Abstract
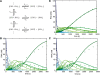
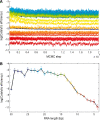
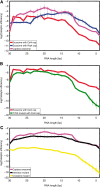
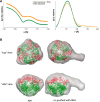
RNA exosomes are large multisubunit assemblies involved in controlled RNA processing. The archaeal exosome possesses a heterohexameric processing chamber with three RNase-PH-like active sites, capped by Rrp4- or Csl4-type subunits containing RNA-binding domains. RNA degradation by RNA exosomes has not been studied in a quantitative manner because of the complex kinetics involved, and exosome features contributing to efficient RNA degradation remain unclear. Here we derive a quantitative kinetic model for degradation of a model substrate by the archaeal exosome. Markov Chain Monte Carlo methods for parameter estimation allow for the comparison of reaction kinetics between different exosome variants and substrates. We show that long substrates are degraded in a processive and short RNA in a more distributive manner and that the cap proteins influence degradation speed. Our results, supported by small angle X-ray scattering, suggest that the Rrp4-type cap efficiently recruits RNA but prevents fast RNA degradation of longer RNAs by molecular friction, likely by RNA contacts to its unique KH-domain. We also show that formation of the RNase-PH like ring with entrapped RNA is not required for high catalytic efficiency, suggesting that the exosome chamber evolved for controlled processivity, rather than for catalytic chemistry in RNA decay.
Figures


Similar articles
-
RNA channelling by the archaeal exosome.EMBO Rep. 2007 May;8(5):470-6. doi: 10.1038/sj.embor.7400945. Epub 2007 Mar 23. EMBO Rep. 2007. PMID: 17380186 Free PMC article.
-
Structural framework for the mechanism of archaeal exosomes in RNA processing.Mol Cell. 2005 Nov 11;20(3):461-71. doi: 10.1016/j.molcel.2005.10.018. Mol Cell. 2005. PMID: 16285927
-
Rrp4 and Csl4 are needed for efficient degradation but not for polyadenylation of synthetic and natural RNA by the archaeal exosome.Biochemistry. 2008 Dec 16;47(50):13158-68. doi: 10.1021/bi8012214. Biochemistry. 2008. PMID: 19053279
-
The archaeal exosome.Adv Exp Med Biol. 2010;702:29-38. Adv Exp Med Biol. 2010. PMID: 21618872 Review.
-
Structure and function of the archaeal exosome.Wiley Interdiscip Rev RNA. 2014 Sep-Oct;5(5):623-35. doi: 10.1002/wrna.1234. Epub 2014 Apr 30. Wiley Interdiscip Rev RNA. 2014. PMID: 24789718 Review.
Cited by
-
Archaeal DnaG contains a conserved N-terminal RNA-binding domain and enables tailing of rRNA by the exosome.Nucleic Acids Res. 2014 Nov 10;42(20):12691-706. doi: 10.1093/nar/gku969. Epub 2014 Oct 17. Nucleic Acids Res. 2014. PMID: 25326320 Free PMC article.
-
Exosomal Protein Deficiencies: How Abnormal RNA Metabolism Results in Childhood-Onset Neurological Diseases.J Neuromuscul Dis. 2015;2(Suppl 2):S31-S37. doi: 10.3233/JND-150086. Epub 2015 Jul 22. J Neuromuscul Dis. 2015. PMID: 27127732 Free PMC article.
-
Processive RNA decay by the exosome: merits of a quantitative Bayesian sampling approach.RNA Biol. 2011 Jan-Feb;8(1):55-60. doi: 10.4161/rna.8.1.14067. Epub 2011 Jan 1. RNA Biol. 2011. PMID: 21282980 Free PMC article.
-
Crystal structure of Caulobacter crescentus polynucleotide phosphorylase reveals a mechanism of RNA substrate channelling and RNA degradosome assembly.Open Biol. 2012 Apr;2(4):120028. doi: 10.1098/rsob.120028. Open Biol. 2012. PMID: 22724061 Free PMC article.
-
The Rrp4-exosome complex recruits and channels substrate RNA by a unique mechanism.Nat Chem Biol. 2017 May;13(5):522-528. doi: 10.1038/nchembio.2328. Epub 2017 Mar 13. Nat Chem Biol. 2017. PMID: 28288106 Free PMC article.
References
-
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′–>5′ exoribonucleases. Cell. 1997;91:457–466. - PubMed
-
- Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

