Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs
- PMID: 20392839
- PMCID: PMC2877643
- DOI: 10.1091/mbc.e09-09-0801
Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs
Abstract
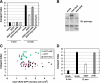
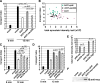
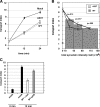
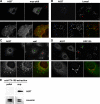
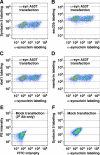
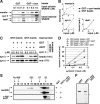
Toxicity of human alpha-synuclein when expressed in simple organisms can be suppressed by overexpression of endoplasmic reticulum (ER)-to-Golgi transport machinery, suggesting that inhibition of constitutive secretion represents a fundamental cause of the toxicity. Whether similar inhibition in mammals represents a cause of familial Parkinson's disease has not been established. We tested elements of this hypothesis by expressing human alpha-synuclein in mammalian kidney and neuroendocrine cells and assessing ER-to-Golgi transport. Overexpression of wild type or the familial disease-associated A53T mutant alpha-synuclein delayed transport by up to 50%; however, A53T inhibited more potently. The secretory delay occurred at low expression levels and was not accompanied by insoluble alpha-synuclein aggregates or mistargeting of transport machinery, suggesting a direct action of soluble alpha-synuclein on trafficking proteins. Co-overexpression of ER/Golgi arginine soluble N-ethylmaleimide-sensitive factor attachment protein receptors (R-SNAREs) specifically rescued transport, indicating that alpha-synuclein antagonizes SNARE function. Ykt6 reversed alpha-synuclein inhibition much more effectively than sec22b, suggesting a possible neuroprotective role for the enigmatic high expression of ykt6 in neurons. In in vitro reconstitutions, purified alpha-synuclein A53T protein specifically inhibited COPII vesicle docking and fusion at a pre-Golgi step. Finally, soluble alpha-synuclein A53T directly bound ER/Golgi SNAREs and inhibited SNARE complex assembly, providing a potential mechanism for toxic effects in the early secretory pathway.
Figures



Similar articles
-
Sec24C/D-isoform-specific sorting of the preassembled ER-Golgi Q-SNARE complex.Mol Biol Cell. 2016 Sep 1;27(17):2697-707. doi: 10.1091/mbc.E16-04-0229. Epub 2016 Jul 13. Mol Biol Cell. 2016. PMID: 27413010 Free PMC article.
-
Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport.J Biol Chem. 2001 Jul 20;276(29):27480-7. doi: 10.1074/jbc.M102786200. Epub 2001 Apr 25. J Biol Chem. 2001. PMID: 11323436
-
Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment.Mol Biol Cell. 1999 Feb;10(2):435-53. doi: 10.1091/mbc.10.2.435. Mol Biol Cell. 1999. PMID: 9950687 Free PMC article.
-
COPII and COPI traffic at the ER-Golgi interface.Physiology (Bethesda). 2011 Oct;26(5):348-64. doi: 10.1152/physiol.00017.2011. Physiology (Bethesda). 2011. PMID: 22013193 Review.
-
Biogenesis of ER-to-Golgi transport carriers: complex roles of COPII in ER export.Trends Cell Biol. 2004 Feb;14(2):57-61. doi: 10.1016/j.tcb.2003.12.001. Trends Cell Biol. 2004. PMID: 15106609 Review.
Cited by
-
α-Synuclein in Parkinson's disease.Cold Spring Harb Perspect Med. 2012 Feb;2(2):a009399. doi: 10.1101/cshperspect.a009399. Cold Spring Harb Perspect Med. 2012. PMID: 22355802 Free PMC article. Review.
-
Alpha-Synuclein FRET Biosensors Reveal Early Alpha-Synuclein Aggregation in the Endoplasmic Reticulum.Life (Basel). 2020 Aug 11;10(8):147. doi: 10.3390/life10080147. Life (Basel). 2020. PMID: 32796544 Free PMC article.
-
Stx5-Mediated ER-Golgi Transport in Mammals and Yeast.Cells. 2019 Jul 26;8(8):780. doi: 10.3390/cells8080780. Cells. 2019. PMID: 31357511 Free PMC article. Review.
-
Mammalian ribosomal and chaperone protein RPS3A counteracts α-synuclein aggregation and toxicity in a yeast model system.Biochem J. 2013 Nov 1;455(3):295-306. doi: 10.1042/BJ20130417. Biochem J. 2013. PMID: 23924367 Free PMC article.
-
Examining the Toxicity of α-Synuclein in Neurodegenerative Disorders.Life (Basel). 2021 Oct 22;11(11):1126. doi: 10.3390/life11111126. Life (Basel). 2021. PMID: 34833002 Free PMC article. Review.
References
-
- Bentley M., Liang Y., Mullen K., Xu D., Sztul E., Hay J. C. SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J. Biol. Chem. 2006;281:38825–38833. - PubMed
-
- Cai H., Yu S., Menon S., Cai Y., Lazarova D., Fu C., Reinisch K., Hay J. C., Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. - PubMed
-
- Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O. M., Südhof T. C. alpha-Synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

