Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs
- PMID: 20392839
- PMCID: PMC2877643
- DOI: 10.1091/mbc.e09-09-0801
Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs
Abstract
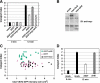
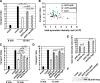
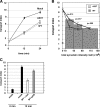
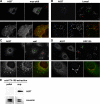
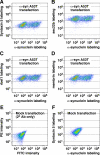
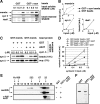
Toxicity of human alpha-synuclein when expressed in simple organisms can be suppressed by overexpression of endoplasmic reticulum (ER)-to-Golgi transport machinery, suggesting that inhibition of constitutive secretion represents a fundamental cause of the toxicity. Whether similar inhibition in mammals represents a cause of familial Parkinson's disease has not been established. We tested elements of this hypothesis by expressing human alpha-synuclein in mammalian kidney and neuroendocrine cells and assessing ER-to-Golgi transport. Overexpression of wild type or the familial disease-associated A53T mutant alpha-synuclein delayed transport by up to 50%; however, A53T inhibited more potently. The secretory delay occurred at low expression levels and was not accompanied by insoluble alpha-synuclein aggregates or mistargeting of transport machinery, suggesting a direct action of soluble alpha-synuclein on trafficking proteins. Co-overexpression of ER/Golgi arginine soluble N-ethylmaleimide-sensitive factor attachment protein receptors (R-SNAREs) specifically rescued transport, indicating that alpha-synuclein antagonizes SNARE function. Ykt6 reversed alpha-synuclein inhibition much more effectively than sec22b, suggesting a possible neuroprotective role for the enigmatic high expression of ykt6 in neurons. In in vitro reconstitutions, purified alpha-synuclein A53T protein specifically inhibited COPII vesicle docking and fusion at a pre-Golgi step. Finally, soluble alpha-synuclein A53T directly bound ER/Golgi SNAREs and inhibited SNARE complex assembly, providing a potential mechanism for toxic effects in the early secretory pathway.
Figures



References
-
- Bentley M., Liang Y., Mullen K., Xu D., Sztul E., Hay J. C. SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J. Biol. Chem. 2006;281:38825–38833. - PubMed
-
- Cai H., Yu S., Menon S., Cai Y., Lazarova D., Fu C., Reinisch K., Hay J. C., Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. - PubMed
-
- Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O. M., Südhof T. C. alpha-Synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

