DDX6 (Rck/p54) is required for efficient hepatitis C virus replication but not for internal ribosome entry site-directed translation
- PMID: 20392846
- PMCID: PMC2903299
- DOI: 10.1128/JVI.00397-10
DDX6 (Rck/p54) is required for efficient hepatitis C virus replication but not for internal ribosome entry site-directed translation
Abstract
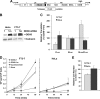
DDX6 (Rck/p54) is an evolutionarily conserved member of the SF2 DEAD-box RNA helicase family that contributes to the regulation of translation and storage and the degradation of cellular mRNAs. It interacts with multiple proteins and is a component of the micro-RNA (miRNA)-induced silencing complex (miRISC). Since miRNA-122 (miR-122) is essential for efficient hepatitis C virus (HCV) replication, we investigated the requirement for DDX6 in HCV replication in cultured hepatoma cells. Small interfering RNA (siRNA)-mediated knockdown of DDX6 and rescue with an siRNA-resistant mutant demonstrated that DDX6 expression is indeed required for optimal HCV replication. However, DDX6 knockdown did not impair miR-122 biogenesis or alter HCV responsiveness to miR-122 supplementation. Overexpression of DDX6 fused to EYFP (EYFP-DDX6) enhanced replication, whereas a helicase-deficient mutant with a substitution in the conserved DEAD-box motif II (DQAD) had a dominant-negative effect, reducing HCV yields. Coimmunoprecipitation experiments revealed an intracellular complex containing DDX6, HCV core protein, and both viral and cellular RNAs, the formation of which was dependent upon the C-terminal domain of DDX6 but not DDX6 helicase activity. However, since DDX6 abundance influenced the replication of subgenomic HCV RNAs lacking core sequence, the relevance of this complex is uncertain. Importantly, DDX6 knockdown caused minimal reductions in cellular proliferation, generally stimulated cellular translation ([(35)S]Met incorporation), and did not impair translation directed by the HCV internal ribosome entry site. Thus, DDX6 helicase activity is essential for efficient HCV replication, reflecting essential roles for DDX6 in HCV genome amplification and/or maintenance of cellular homeostasis.
Figures

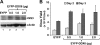

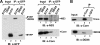


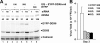


Similar articles
-
Modulation of hepatitis C virus RNA accumulation and translation by DDX6 and miR-122 are mediated by separate mechanisms.PLoS One. 2013 Jun 24;8(6):e67437. doi: 10.1371/journal.pone.0067437. Print 2013. PLoS One. 2013. PMID: 23826300 Free PMC article.
-
Cellular DEAD-box RNA helicase DDX6 modulates interaction of miR-122 with the 5' untranslated region of hepatitis C virus RNA.Virology. 2017 Jul;507:231-241. doi: 10.1016/j.virol.2017.04.014. Epub 2017 Apr 26. Virology. 2017. PMID: 28456022 Free PMC article.
-
DDX6 post-transcriptionally down-regulates miR-143/145 expression through host gene NCR143/145 in cancer cells.Biochim Biophys Acta. 2013 Oct;1829(10):1102-10. doi: 10.1016/j.bbagrm.2013.07.010. Epub 2013 Aug 9. Biochim Biophys Acta. 2013. PMID: 23932921
-
DDX6 and its orthologs as modulators of cellular and viral RNA expression.Wiley Interdiscip Rev RNA. 2014 Sep-Oct;5(5):659-78. doi: 10.1002/wrna.1237. Epub 2014 Apr 30. Wiley Interdiscip Rev RNA. 2014. PMID: 24788243 Review.
-
Understanding the interaction of hepatitis C virus with host DEAD-box RNA helicases.World J Gastroenterol. 2014 Mar 21;20(11):2913-26. doi: 10.3748/wjg.v20.i11.2913. World J Gastroenterol. 2014. PMID: 24659882 Free PMC article. Review.
Cited by
-
miR-122 and the Hepatitis C RNA genome: more than just stability.RNA Biol. 2013 Jun;10(6):919-23. doi: 10.4161/rna.25137. Epub 2013 May 22. RNA Biol. 2013. PMID: 23770926 Free PMC article.
-
DDX RNA helicases: key players in cellular homeostasis and innate antiviral immunity.J Virol. 2024 Oct 22;98(10):e0004024. doi: 10.1128/jvi.00040-24. Epub 2024 Aug 30. J Virol. 2024. PMID: 39212449 Free PMC article. Review.
-
Use of Cellular Decapping Activators by Positive-Strand RNA Viruses.Viruses. 2016 Dec 21;8(12):340. doi: 10.3390/v8120340. Viruses. 2016. PMID: 28009841 Free PMC article. Review.
-
Regulation of Hepatitis C Virus Genome Replication by Xrn1 and MicroRNA-122 Binding to Individual Sites in the 5' Untranslated Region.J Virol. 2015 Jun;89(12):6294-311. doi: 10.1128/JVI.03631-14. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855736 Free PMC article.
-
Regulation of stress granules and P-bodies during RNA virus infection.Wiley Interdiscip Rev RNA. 2013 May-Jun;4(3):317-31. doi: 10.1002/wrna.1162. Epub 2013 Apr 3. Wiley Interdiscip Rev RNA. 2013. PMID: 23554219 Free PMC article. Review.
References
-
- Akao, Y., H. Yoshida, K. Matsumoto, T. Matsui, K. Hogetu, N. Tanaka, and J. Usukura. 2003. A tumour-associated DEAD-box protein, rck/p54 exhibits RNA unwinding activity toward c-myc RNAs in vitro. Genes Cells 8:671-676. - PubMed
-
- Angus, A. G., D. Dalrymple, S. Boulant, D. R. McGivern, R. F. Clayton, M. J. Scott, R. Adair, S. Graham, A. M. Owsianka, P. Targett-Adams, K. Li, T. Wakita, J. McLauchlan, S. M. Lemon, and A. H. Patel. 2010. Requirement of cellular DDX3 for hepatitis C virus replication is unrelated to its interaction with the viral core protein. J. Gen. Virol. 91:122-132. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

