Structure/Function analysis of the vaccinia virus F18 phosphoprotein, an abundant core component required for virion maturation and infectivity
- PMID: 20392848
- PMCID: PMC2903294
- DOI: 10.1128/JVI.00399-10
Structure/Function analysis of the vaccinia virus F18 phosphoprotein, an abundant core component required for virion maturation and infectivity
Abstract
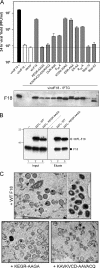
Poxvirus virions, whose outer membrane surrounds two lateral bodies and a core, contain at least 70 different proteins. The F18 phosphoprotein is one of the most abundant core components and is essential for the assembly of mature virions. We report here the results of a structure/function analysis in which the role of conserved cysteine residues, clusters of charged amino acids and clusters of hydrophobic/aromatic amino acids have been assessed. Taking advantage of a recombinant virus in which F18 expression is IPTG (isopropyl-beta-d-thiogalactopyranoside) dependent, we developed a transient complementation assay to evaluate the ability of mutant alleles of F18 to support virion morphogenesis and/or to restore the production of infectious virus. We have also examined protein-protein interactions, comparing the ability of mutant and WT F18 proteins to interact with WT F18 and to interact with the viral A30 protein, another essential core component. We show that F18 associates with an A30-containing multiprotein complex in vivo in a manner that depends upon clusters of hydrophobic/aromatic residues in the N' terminus of the F18 protein but that it is not required for the assembly of this complex. Finally, we confirmed that two PSSP motifs within F18 are the sites of phosphorylation by cellular proline-directed kinases in vitro and in vivo. Mutation of both of these phosphorylation sites has no apparent impact on virion morphogenesis but leads to the assembly of virions with significantly reduced infectivity.
Figures







Similar articles
-
Genetic and cell biological characterization of the vaccinia virus A30 and G7 phosphoproteins.J Virol. 2005 Jun;79(11):7146-61. doi: 10.1128/JVI.79.11.7146-7161.2005. J Virol. 2005. PMID: 15890954 Free PMC article.
-
Structure-Function Analysis of Two Interacting Vaccinia Proteins That Are Critical for Viral Morphogenesis: L2 and A30.5.J Virol. 2022 Jan 26;96(2):e0157721. doi: 10.1128/JVI.01577-21. Epub 2021 Nov 3. J Virol. 2022. PMID: 34730390 Free PMC article.
-
Vaccinia virus morphogenesis: a13 phosphoprotein is required for assembly of mature virions.J Virol. 2004 Aug;78(16):8885-901. doi: 10.1128/JVI.78.16.8885-8901.2004. J Virol. 2004. PMID: 15280497 Free PMC article.
-
In a nutshell: structure and assembly of the vaccinia virion.Adv Virus Res. 2006;66:31-124. doi: 10.1016/S0065-3527(06)66002-8. Adv Virus Res. 2006. PMID: 16877059 Review.
-
Assembly and Evolution of Poxviruses.Adv Exp Med Biol. 2024;1451:35-54. doi: 10.1007/978-3-031-57165-7_3. Adv Exp Med Biol. 2024. PMID: 38801570 Review.
Cited by
-
mTOR Dysregulation by Vaccinia Virus F17 Controls Multiple Processes with Varying Roles in Infection.J Virol. 2019 Jul 17;93(15):e00784-19. doi: 10.1128/JVI.00784-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118254 Free PMC article.
-
Fine structure of the vaccinia virion determined by controlled degradation and immunolocalization.Virology. 2015 Jan 15;475:204-18. doi: 10.1016/j.virol.2014.11.020. Epub 2014 Dec 8. Virology. 2015. PMID: 25486587 Free PMC article.
-
Isolation and Characterization of vΔI3 Confirm that Vaccinia Virus SSB Plays an Essential Role in Viral Replication.J Virol. 2018 Jan 2;92(2):e01719-17. doi: 10.1128/JVI.01719-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093092 Free PMC article.
-
Poxvirus Interactions with the Host Ubiquitin System.Pathogens. 2021 Aug 16;10(8):1034. doi: 10.3390/pathogens10081034. Pathogens. 2021. PMID: 34451498 Free PMC article. Review.
-
Vaccinia Virus Defective Particles Lacking the F17 Protein Do Not Inhibit Protein Synthesis: F17, a Double-Edged Sword for Protein Synthesis?Int J Mol Sci. 2024 Jan 23;25(3):1382. doi: 10.3390/ijms25031382. Int J Mol Sci. 2024. PMID: 38338659 Free PMC article.
References
-
- Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques 23:1094-1097. - PubMed
-
- Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31-124. - PubMed
-
- Dyster, L. M., and E. G. Niles. 1991. Genetic and biochemical characterization of vaccinia virus genes D2L and D3R which encode virion structural proteins. Virology 182:455-467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

