The RING finger domain of Varicella-Zoster virus ORF61p has E3 ubiquitin ligase activity that is essential for efficient autoubiquitination and dispersion of Sp100-containing nuclear bodies
- PMID: 20392849
- PMCID: PMC2903287
- DOI: 10.1128/JVI.00335-10
The RING finger domain of Varicella-Zoster virus ORF61p has E3 ubiquitin ligase activity that is essential for efficient autoubiquitination and dispersion of Sp100-containing nuclear bodies
Abstract
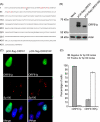
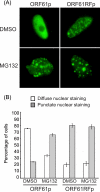
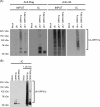
Varicella zoster virus encodes an immediate-early (IE) protein termed ORF61p that is orthologous to the herpes simplex virus IE protein ICP0. Although these proteins share several functional properties, ORF61p does not fully substitute for ICP0. The greatest region of similarity between these proteins is a RING finger domain. We demonstrate that disruption of the ORF61p RING finger domain by amino acid substitution (Cys19Gly) alters ORF61p intranuclear distribution and abolishes ORF61p-mediated dispersion of Sp100-containing nuclear bodies. In addition, we demonstrate that an intact ORF61p RING finger domain is necessary for E3 ubiquitin ligase activity and is required for autoubiquitination and regulation of protein stability.
Figures
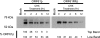
Similar articles
-
Complementation of a herpes simplex virus ICP0 null mutant by varicella-zoster virus ORF61p.J Virol. 2009 Oct;83(20):10637-43. doi: 10.1128/JVI.01144-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656893 Free PMC article.
-
Components of nuclear domain 10 bodies regulate varicella-zoster virus replication.J Virol. 2009 May;83(9):4262-74. doi: 10.1128/JVI.00021-09. Epub 2009 Feb 11. J Virol. 2009. PMID: 19211749 Free PMC article.
-
Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins.J Virol. 2000 Nov;74(21):10006-17. doi: 10.1128/jvi.74.21.10006-10017.2000. J Virol. 2000. PMID: 11024129 Free PMC article.
-
Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins.J Virol. 2010 Apr;84(7):3476-87. doi: 10.1128/JVI.02544-09. Epub 2010 Jan 27. J Virol. 2010. PMID: 20106921 Free PMC article.
-
When MARCH family proteins meet viral infections.Virol J. 2021 Mar 2;18(1):49. doi: 10.1186/s12985-021-01520-4. Virol J. 2021. PMID: 33653359 Free PMC article. Review.
Cited by
-
HSV-1 ICP0: An E3 Ubiquitin Ligase That Counteracts Host Intrinsic and Innate Immunity.Cells. 2014 May 20;3(2):438-54. doi: 10.3390/cells3020438. Cells. 2014. PMID: 24852129 Free PMC article.
-
The Role of ND10 Nuclear Bodies in Herpesvirus Infection: A Frenemy for the Virus?Viruses. 2021 Feb 3;13(2):239. doi: 10.3390/v13020239. Viruses. 2021. PMID: 33546431 Free PMC article. Review.
-
Attenuation of the adaptive immune response in rhesus macaques infected with simian varicella virus lacking open reading frame 61.J Virol. 2013 Feb;87(4):2151-63. doi: 10.1128/JVI.02369-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221560 Free PMC article.
-
PML Body Component Sp100A Restricts Wild-Type Herpes Simplex Virus 1 Infection.J Virol. 2022 Apr 27;96(8):e0027922. doi: 10.1128/jvi.00279-22. Epub 2022 Mar 30. J Virol. 2022. PMID: 35353002 Free PMC article.
-
N-terminal phosphorylation sites of herpes simplex virus 1 ICP0 differentially regulate its activities and enhance viral replication.J Virol. 2013 Feb;87(4):2109-19. doi: 10.1128/JVI.02588-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221554 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

