Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection
- PMID: 20392855
- PMCID: PMC2903281
- DOI: 10.1128/JVI.00225-10
Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection
Abstract
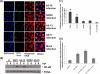
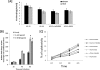
Following virus infection, one of the cellular responses to limit the virus spread is induction of apoptosis. In the present study, we report role of rotavirus nonstructural protein 1 (NSP1) in regulating apoptosis by activating prosurvival pathways such as phosphatidylinositol 3-kinase (PI3K)/Akt and NF-kappaB (nuclear factor kappaB) during early hours of infections (2 to 8 hpi). The NSP1 mutant strain A5-16 induces weak and transient activation of Akt (protein kinase B) and p65 NF-kappaB compared to the isogenic wild-type strain A5-13 in MA104 or HT29 cells. The weak NF-kappaB promoter activity or Akt phosphorylation after A5-16 infection could be complemented in cells transfected with plasmid expressing NSP1 after infection with the rotavirus A5-16 strain. In cells either infected with A5-13 or transfected with pcD-NSP1, coimmunoprecipitation of NSP1 with phosphoinositide 3-kinase (PI3K) was observed, indicating that strong activation of PI3K/Akt could be due to its interaction with NSP1. In addition, after infection with same multiplicity of infection, A5-16 showed reduced number of viral particles compared to the A5-13 strain at the end of the replication cycle. A lower growth rate could be due to weak induction of PI3K/Akt and NF-kappaB, since the A5-13 strain also showed reduced growth in the presence of PI3K or NF-kappaB inhibitors. This effect was interferon independent; however, it was partly due to significantly higher caspase-3 activity, poly-ADP ribose polymerase (PARP) cleavage, and apoptosis during earlier stages of infection with the NSP1 mutant. Thus, our data suggest that NSP1 positively supports rotavirus growth by suppression of premature apoptosis for improved virus growth after infection.
Figures




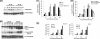
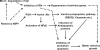
References
-
- Aksoy, E., W. Vanden Berghe, S. Detienne, Z. Amraoui, K. A. Fitzgerald, G. Haegeman, M. Goldman, and F. Willems. 2005. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-κB activation and IFN-β synthesis downstream of Toll-like receptor 3 and 4. Eur. J. Immunol. 35:2200-2209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

