Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant
- PMID: 20392857
- PMCID: PMC2876657
- DOI: 10.1128/JVI.02533-09
Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant
Abstract
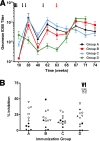
We have previously shown that rhesus macaques were partially protected against high-dose intravenous challenge with simian-human immunodeficiency virus SHIV(SF162P4) following sequential immunization with alphavirus replicon particles (VRP) of a chimeric recombinant VEE/SIN alphavirus (derived from Venezuelan equine encephalitis virus [VEE] and the Sindbis virus [SIN]) encoding human immunodeficiency virus type 1 HIV-1(SF162) gp140DeltaV2 envelope (Env) and trimeric Env protein in MF59 adjuvant (R. Xu, I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos, AIDS Res. Hum. Retroviruses 22:1022-1030, 2006). The protection did not require T-cell immune responses directed toward simian immunodeficiency virus (SIV) Gag. We extend those findings here to demonstrate antibody-mediated protection against mucosal challenge in macaques using prime-boost regimens incorporating both intramuscular and mucosal routes of delivery. The macaques in the vaccination groups were primed with VRP and then boosted with Env protein in MF59 adjuvant, or they were given VRP intramuscular immunizations alone and then challenged with SHIV(SF162P4) (intrarectal challenge). The results demonstrated that these vaccines were able to effectively protect the macaques to different degrees against subsequent mucosal SHIV challenge, but most noteworthy, all macaques that received the intramuscular VRP prime plus Env protein boost were completely protected. A statistically significant association was observed between the titer of virus neutralizing and binding antibodies as well as the avidity of anti-Env antibodies measured prechallenge and protection from infection. These results highlight the merit of the alphavirus replicon vector prime plus Env protein boost vaccine approach for the induction of protective antibody responses and are of particular relevance to advancing our understanding of the potential correlates of immune protection against HIV infection at a relevant mucosal portal of entry.
Figures



References
-
- Barnett, S. W., J. M. Klinger, B. Doe, C. M. Walker, L. Hansen, A. M. Duliege, and F. M. Sinangil. 1998. Prime-boost immunization strategies against HIV. AIDS Res. Hum. Retroviruses 14(Suppl. 3):S299-S309. - PubMed
-
- Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22:339-348. - PubMed
-
- Baroncelli, S., D. R. Negri, Z. Michelini, and A. Cara. 2008. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Rev. Vaccines 7:1419-1434. - PubMed
-
- Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A. 97:4192-4197. - PMC - PubMed
-
- Barouch, D. H., J. Liu, D. M. Lynch, K. L. O'Brien, A. La Porte, N. L. Simmons, A. M. Riggs, S. Clark, P. Abbink, D. C. Montefiori, G. Landucci, D. N. Forthal, S. G. Self, A. Carville, K. Mansfield, and J. Goudsmit. 2009. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 83:9584-9590. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

