Binding to gangliosides containing N-acetylneuraminic acid is sufficient to mediate the immunomodulatory properties of the nontoxic mucosal adjuvant LT-IIb(T13I)
- PMID: 20392887
- PMCID: PMC2884423
- DOI: 10.1128/CVI.00076-10
Binding to gangliosides containing N-acetylneuraminic acid is sufficient to mediate the immunomodulatory properties of the nontoxic mucosal adjuvant LT-IIb(T13I)
Abstract
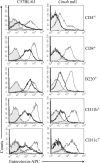
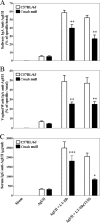
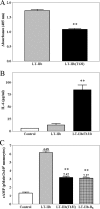
By use of a mouse mucosal immunization model, LT-IIb(T13I), a nontoxic mutant type II heat-labile enterotoxin, was shown to have potent mucosal and systemic adjuvant properties. In contrast to LT-IIb, which binds strongly to ganglioside receptors decorated with either N-acetylneuraminic acid (NeuAc) or N-glycolylneuraminic acid (NeuGc), LT-IIb(T13I) binds NeuAc gangliosides much less well. Rather, LT-IIb(T13I) binds preferentially to NeuGc gangliosides. To determine if the adjuvant properties of LT-IIb(T13I) are altered in the absence of NeuGc ganglioside receptors, experiments were conducted using a Cmah-null mouse line which is deficient in the synthesis of NeuGc gangliosides. Several immunomodulatory properties of LT-IIb(T13I) were shown to be dependent on NeuGc gangliosides. LT-IIb(T13I) had reduced binding activity for NeuGc-deficient B cells and macrophages; binding to NeuGc-deficient T cells and dendritic cells (DC) was essentially undetectable. Treatment of Cmah-null macrophages with LT-IIb(T13I), however, upregulated the transcription of interleukin-4 (IL-4), IL-6, IL-17, and gamma interferon (IFN-gamma), four cytokines important for promoting immune responses. The production of mucosal IgA and serum IgG against an immunizing antigen was augmented in NeuGc-deficient mice administered LT-IIb(T13I) as a mucosal adjuvant. Notably, NeuGc gangliosides are not expressed in humans. Still, treatment of human monocytes with LT-IIb(T13I) induced the secretion of IL-6, an inflammatory cytokine that mediates differential control of leukocyte activation. These results suggested that NeuAc gangliosides are sufficient to mediate the immunomodulatory properties of LT-IIb(T13I) in mice and in human cells. The nontoxic mutant enterotoxin LT-IIb(T13I), therefore, is potentially a new and safe human mucosal adjuvant.
Figures

Similar articles
-
Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities.Infect Immun. 2005 Mar;73(3):1330-42. doi: 10.1128/IAI.73.3.1330-1342.2005. Infect Immun. 2005. PMID: 15731030 Free PMC article.
-
Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I).Glycobiology. 2010 Jan;20(1):41-54. doi: 10.1093/glycob/cwp141. Epub 2009 Sep 12. Glycobiology. 2010. PMID: 19749203 Free PMC article.
-
LT-IIb(T13I), a non-toxic type II heat-labile enterotoxin, augments the capacity of a ricin toxin subunit vaccine to evoke neutralizing antibodies and protective immunity.PLoS One. 2013 Aug 2;8(8):e69678. doi: 10.1371/journal.pone.0069678. Print 2013. PLoS One. 2013. PMID: 23936344 Free PMC article.
-
Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins.Expert Rev Vaccines. 2007 Oct;6(5):821-34. doi: 10.1586/14760584.6.5.821. Expert Rev Vaccines. 2007. PMID: 17931161 Free PMC article. Review.
-
The role of ADP-ribosylation and G(M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin.Immunol Cell Biol. 1998 Jun;76(3):270-9. doi: 10.1046/j.1440-1711.1998.00745.x. Immunol Cell Biol. 1998. PMID: 9682971 Review.
Cited by
-
LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a nonmammalian host.Infect Immun. 2010 Nov;78(11):4705-13. doi: 10.1128/IAI.00730-10. Epub 2010 Aug 16. Infect Immun. 2010. PMID: 20713622 Free PMC article.
-
LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb.Vaccine. 2011 Jan 17;29(4):721-7. doi: 10.1016/j.vaccine.2010.11.020. Epub 2010 Nov 21. Vaccine. 2011. PMID: 21095251 Free PMC article.
-
Structure-activity correlations of variant forms of the B pentamer of Escherichia coli type II heat-labile enterotoxin LT-IIb with Toll-like receptor 2 binding.Acta Crystallogr D Biol Crystallogr. 2012 Dec;68(Pt 12):1604-12. doi: 10.1107/S0907444912038917. Epub 2012 Nov 9. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 23151625 Free PMC article.
-
A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation.Toxins (Basel). 2024 Jul 11;16(7):311. doi: 10.3390/toxins16070311. Toxins (Basel). 2024. PMID: 39057951 Free PMC article.
-
Animal Enterotoxigenic Escherichia coli.EcoSal Plus. 2016 Oct;7(1):10.1128/ecosalplus.ESP-0006-2016. doi: 10.1128/ecosalplus.ESP-0006-2016. EcoSal Plus. 2016. PMID: 27735786 Free PMC article. Review.
References
-
- Arce, S., H. F. Nawar, G. Muehlinghaus, M. W. Russell, and T. D. Connell. 2007. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect. Immun. 75:1413-1423. - PMC - PubMed
-
- Armstrong, M. E., E. C. Lavelle, C. E. Loscher, M. A. Lynch, and K. H. Mills. 2005. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. J. Infect. Dis. 192:1628-1633. - PubMed
-
- Belardelli, F. 1995. Role of interferons and other cytokines in the regulation of the immune response. APMIS 103:161-179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

