Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy
- PMID: 20392939
- PMCID: PMC2874941
- DOI: 10.1523/JNEUROSCI.5591-09.2010
Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy
Abstract
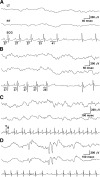
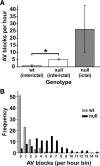
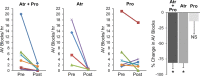
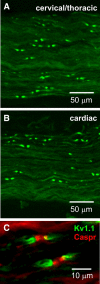
Mice lacking Kv1.1 Shaker-like potassium channels encoded by the Kcna1 gene exhibit severe seizures and die prematurely. The channel is widely expressed in brain but only minimally, if at all, in mouse myocardium. To test whether Kv1.1-potassium deficiency could underlie primary neurogenic cardiac dysfunction, we performed simultaneous video EEG-ECG recordings and found that Kcna1-null mice display potentially malignant interictal cardiac abnormalities, including a fivefold increase in atrioventricular (AV) conduction blocks, as well as bradycardia and premature ventricular contractions. During seizures the occurrence of AV conduction blocks increased, predisposing Kv1.1-deficient mice to sudden unexplained death in epilepsy (SUDEP), which we recorded fortuitously in one animal. To determine whether the interictal AV conduction blocks were of cardiac or neural origin, we examined their response to selective pharmacological blockade of the autonomic nervous system. Simultaneous administration of atropine and propranolol to block parasympathetic and sympathetic branches, respectively, eliminated conduction blocks. When administered separately, only atropine ameliorated AV conduction blocks, indicating that excessive parasympathetic tone contributes to the neurocardiac defect. We found no changes in Kv1.1-deficient cardiac structure, but extensive Kv1.1 expression in juxtaparanodes of the wild-type vagus nerve, the primary source of parasympathetic input to the heart, suggesting a novel site of action leading to Kv1.1-associated cardiac bradyarrhythmias. Together, our data suggest that Kv1.1 deficiency leads to impaired neural control of cardiac rhythmicity due in part to aberrant parasympathetic neurotransmission, making Kcna1 a strong candidate gene for human SUDEP.
Figures

Comment in
-
Highlights in basic autonomic neurosciences: epileptic seizure and sudden death.Auton Neurosci. 2012 Apr 3;167(1-2):1-3. doi: 10.1016/j.autneu.2012.01.002. Epub 2012 Jan 28. Auton Neurosci. 2012. PMID: 22284814 No abstract available.
References
-
- Adelman JP, Bond CT, Pessia M, Maylie J. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 1995;15:1449–1454. - PubMed
-
- Altenmüller DM, Zehender M, Schulze-Bonhage A. High-grade atrioventricular block triggered by spontaneous and stimulation-induced epileptic activity in the left temporal lobe. Epilepsia. 2004;45:1640–1644. - PubMed
-
- Berry D, Ayers G. Symmetrized percent change for treatment comparisons. Am Stat. 2006;60:27–31.
-
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. - PubMed
-
- Bird JM, Dembny KAT, Sandeman D, Butler S. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia. 1997;38(Suppl 11):S52–S56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
