Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory
- PMID: 20392949
- PMCID: PMC6632746
- DOI: 10.1523/JNEUROSCI.4209-09.2010
Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory
Abstract
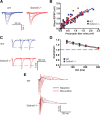
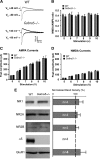
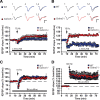
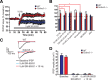
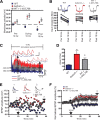
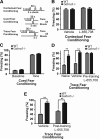
Synaptic plasticity, which is the neuronal substrate for many forms of hippocampus-dependent learning, is attenuated by GABA type A receptor (GABA(A)R)-mediated inhibition. The prevailing notion is that a synaptic or phasic form of GABAergic inhibition regulates synaptic plasticity; however, little is known about the role of GABA(A)R subtypes that generate a tonic or persistent inhibitory conductance. We studied the regulation of synaptic plasticity by alpha5 subunit-containing GABA(A)Rs (alpha5GABA(A)Rs), which generate a tonic inhibitory conductance in CA1 pyramidal neurons using electrophysiological recordings of field and whole-cell potentials in hippocampal slices from both wild-type and null mutant mice for the alpha5 subunit of the GABA(A)R (Gabra5(-/-) mice). In addition, the strength of fear-associated memory was studied. The results showed that alpha5GABA(A)R activity raises the threshold for induction of long-term potentiation in a highly specific band of stimulation frequencies (10-20 Hz) through mechanisms that are predominantly independent of inhibitory synaptic transmission. The deletion or pharmacological inhibition of alpha5GABA(A)Rs caused no change in baseline membrane potential or input resistance but increased depolarization during 10 Hz stimulation. The encoding of hippocampus-dependent memory was regulated by alpha5GABA(A)Rs but only under specific conditions that generate moderate but not robust forms of fear-associated learning. Thus, under specific conditions, alpha5GABA(A)R activity predominates over synaptic inhibition in modifying the strength of both synaptic plasticity in vitro and certain forms of memory in vivo.
Figures

References
-
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternack M. GABA receptor ρ subunit expression in the developing rat brain. Brain Res Dev Brain Res. 2005;154:15–23. - PubMed
-
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006a;51:1023–1029. - PubMed
-
- Atack JR, Pike A, Clarke A, Cook SM, Sohal B, McKernan RM, Dawson GR. Rat pharmacokinetics and pharmacodynamics of a sustained release formulation of the GABAA α5-selective compound L-655,708. Drug Metab Dispos. 2006b;34:887–893. - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. - PubMed
-
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, Gasser R, Moreau JL, Wettstein JG, Buettelmann B, Knust H, Thomas AW, Trube G, Hernandez MC. RO4938581, a novel cognitive enhancer acting at GABAA α5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
