Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord
- PMID: 20392950
- PMCID: PMC6632755
- DOI: 10.1523/JNEUROSCI.3873-09.2010
Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord
Abstract
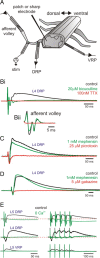
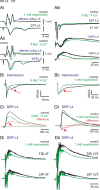
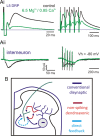
Primary afferent neurotransmission is the fundamental first step in the central processing of sensory stimuli. A major mechanism producing afferent presynaptic inhibition is via a channel-mediated depolarization of their intraspinal terminals which can be recorded extracellularly as a dorsal root potential (DRP). Based on measures of DRP latency it has been inferred that this primary afferent depolarization (PAD) of low-threshold afferents is mediated by minimally trisynaptic pathways with GABAergic interneurons forming last-order axoaxonic synapses onto afferent terminals. We used an in vitro rat spinal cord preparation under conditions that restrict synaptic transmission to test whether more direct low-threshold pathways can produce PAD. Mephenesin or high divalent cation solutions were used to limit oligosynaptic transmission. Recordings of synaptic currents in dorsal horn neurons and population synaptic potentials in ventral roots provided evidence that conventional transmission was chiefly restricted to monosynaptic actions. Under these conditions, DRP amplitude was largely unchanged but with faster time to peak and reduced duration. Similar results were obtained following stimulation of peripheral nerves. Even following near complete block of transmission with high Mg(2+)/low Ca(2+)-containing solution, the evoked DRP was reduced but not blocked. In comparison, in nominally Ca(2+)-free or EGTA-containing solution, the DRP was completely blocked confirming that Ca(2+) entry mediated synaptic transmission is required for DRP genesis. Overall these results demonstrate that PAD of low-threshold primary afferents can occur by more direct synaptic mechanisms, including the possibility of direct negative-feedback or nonspiking dendroaxonic pathways.
Figures
Similar articles
-
Extrasynaptic α5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord.J Neurophysiol. 2018 Dec 1;120(6):2953-2974. doi: 10.1152/jn.00499.2018. Epub 2018 Sep 26. J Neurophysiol. 2018. PMID: 30256739 Free PMC article.
-
Repetitive stimulation induced potentiation of excitatory transmission in the rat dorsal horn: an in vitro study.J Neurophysiol. 1994 Jan;71(1):216-28. doi: 10.1152/jn.1994.71.1.216. J Neurophysiol. 1994. PMID: 7908954
-
Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord.PLoS One. 2014 Feb 28;9(2):e89999. doi: 10.1371/journal.pone.0089999. eCollection 2014. PLoS One. 2014. PMID: 24587177 Free PMC article.
-
Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn.J Neurosci. 2004 May 19;24(20):4749-57. doi: 10.1523/JNEUROSCI.5211-03.2004. J Neurosci. 2004. PMID: 15152035 Free PMC article.
-
Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn.Prog Neurobiol. 1993 Aug;41(2):125-56. doi: 10.1016/0301-0082(93)90006-e. Prog Neurobiol. 1993. PMID: 8392739 Review. No abstract available.
Cited by
-
Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord.J Physiol. 2012 Aug 15;590(16):3821-39. doi: 10.1113/jphysiol.2011.225987. Epub 2012 May 21. J Physiol. 2012. PMID: 22615430 Free PMC article.
-
An in vitro adult mouse muscle-nerve preparation for studying the firing properties of muscle afferents.J Vis Exp. 2014 Sep 24;(91):51948. doi: 10.3791/51948. J Vis Exp. 2014. PMID: 25285602 Free PMC article.
-
Distinct Modes of Presynaptic Inhibition of Cutaneous Afferents and Their Functions in Behavior.Neuron. 2019 Apr 17;102(2):420-434.e8. doi: 10.1016/j.neuron.2019.02.002. Epub 2019 Feb 27. Neuron. 2019. PMID: 30826183 Free PMC article.
-
Neural circuit flexibility in a small sensorimotor system.Curr Opin Neurobiol. 2011 Aug;21(4):544-52. doi: 10.1016/j.conb.2011.05.019. Epub 2011 Jun 30. Curr Opin Neurobiol. 2011. PMID: 21689926 Free PMC article. Review.
-
Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn.Ann N Y Acad Sci. 2013 Mar;1279:90-6. doi: 10.1111/nyas.12056. Ann N Y Acad Sci. 2013. PMID: 23531006 Free PMC article. Review.
References
-
- Alvarez FJ. Anatomical basis for presynaptic inhibition of primary sensory fibers. In: Rudomin P, Romo R, Mendell L, editors. Presynaptic inhibition and neural control. New York: Oxford UP; 1998. pp. 13–49.
-
- Baldissera F, Hultborn H, Illert M. Handbook of physiology, Section I: The nervous system. Baltimore: Williams & Wilkins; 1981. Integration in spinal neuronal systems; pp. 509–595.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
