RIM1alpha and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia
- PMID: 20392954
- PMCID: PMC2860606
- DOI: 10.1523/JNEUROSCI.0328-10.2010
RIM1alpha and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia
Abstract
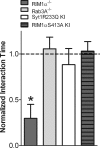
Several presynaptic proteins involved in neurotransmitter release in the CNS have been implicated in schizophrenia in human clinical genetic studies, in postmortem studies, and in studies of putative animal models of schizophrenia. The presynaptic protein RIM1alpha mediates presynaptic plasticity and cognitive function. We now demonstrate that mice deficient in RIM1alpha exhibit abnormalities in multiple schizophrenia-relevant behavioral tasks including prepulse inhibition, response to psychotomimetic drugs, and social interaction. These schizophrenia-relevant behavioral findings are relatively selective to RIM1alpha-deficient mice, as mice bearing mutations in the RIM1alpha binding partners Rab3A or synaptotagmin 1 only show decreased prepulse inhibition. In addition to RIM1alpha's involvement in multiple behavioral abnormalities, these data suggest that alterations in presynaptic forms of short-term plasticity are linked to alterations in prepulse inhibition, a measure of sensorimotor gating.
Figures



Similar articles
-
RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14680-5. doi: 10.1073/pnas.0806679105. Epub 2008 Sep 17. Proc Natl Acad Sci U S A. 2008. PMID: 18799741 Free PMC article.
-
RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone.Nature. 2002 Jan 17;415(6869):321-6. doi: 10.1038/415321a. Nature. 2002. PMID: 11797009
-
The presynaptic active zone protein RIM1α controls epileptogenesis following status epilepticus.J Neurosci. 2012 Sep 5;32(36):12384-95. doi: 10.1523/JNEUROSCI.0223-12.2012. J Neurosci. 2012. PMID: 22956829 Free PMC article.
-
RIM function in short- and long-term synaptic plasticity.Biochem Soc Trans. 2005 Dec;33(Pt 6):1345-9. doi: 10.1042/BST0331345. Biochem Soc Trans. 2005. PMID: 16246115 Review.
-
Gene targeting of presynaptic proteins in synaptic plasticity and memory: across the great divide.Neurobiol Learn Mem. 2006 Jan;85(1):2-15. doi: 10.1016/j.nlm.2005.08.014. Epub 2005 Oct 14. Neurobiol Learn Mem. 2006. PMID: 16230036 Free PMC article. Review.
Cited by
-
Pushing synaptic vesicles over the RIM.Cell Logist. 2011 May;1(3):106-110. doi: 10.4161/cl.1.3.16429. Cell Logist. 2011. PMID: 21922075 Free PMC article.
-
Presynaptic Inhibition Selectively Gates Auditory Transmission to the Brainstem Startle Circuit.Curr Biol. 2018 Aug 20;28(16):2527-2535.e8. doi: 10.1016/j.cub.2018.06.020. Epub 2018 Aug 2. Curr Biol. 2018. PMID: 30078569 Free PMC article.
-
Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission.J Neurosci. 2013 Nov 20;33(47):18448-68. doi: 10.1523/JNEUROSCI.3017-13.2013. J Neurosci. 2013. PMID: 24259569 Free PMC article.
-
Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons.Nat Commun. 2018 Jun 11;9(1):2267. doi: 10.1038/s41467-018-04672-0. Nat Commun. 2018. PMID: 29891949 Free PMC article.
-
Effects of heterozygous deletion of autism-related gene Cullin-3 in mice.PLoS One. 2023 Jul 10;18(7):e0283299. doi: 10.1371/journal.pone.0283299. eCollection 2023. PLoS One. 2023. PMID: 37428799 Free PMC article.
References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Autism Genome Project Consortium. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. - PMC - PubMed
-
- Blennow K, Bogdanovic N, Gottfries CG, Davidsson P. The growth-associated protein GAP-43 is increased in the hippocampus and in the gyrus cinguli in schizophrenia. J Mol Neurosci. 1999;13:101–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
