Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons
- PMID: 20392955
- PMCID: PMC2919857
- DOI: 10.1523/JNEUROSCI.5963-09.2010
Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons
Abstract
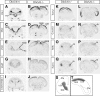
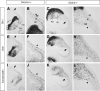
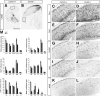
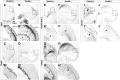
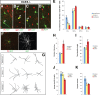
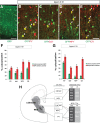
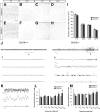
Dlx5 and Dlx6 homeobox genes are expressed in developing and mature cortical interneurons. Simultaneous deletion of Dlx5 and 6 results in exencephaly of the anterior brain; despite this defect, prenatal basal ganglia differentiation appeared largely intact, while tangential migration of Lhx6(+) and Mafb(+) interneurons to the cortex was reduced and disordered. The migration deficits were associated with reduced CXCR4 expression. Transplantation of mutant immature interneurons into a wild-type brain demonstrated that loss of either Dlx5 or Dlx5&6 preferentially reduced the number of mature parvalbumin(+) interneurons; those parvalbumin(+) interneurons that were present had increased dendritic branching. Dlx5/6(+/-) mice, which appear normal histologically, show spontaneous electrographic seizures and reduced power of gamma oscillations. Thus, Dlx5&6 appeared to be required for development and function of somal innervating (parvalbumin(+)) neocortical interneurons. This contrasts with Dlx1, whose function is required for dendrite innervating (calretinin(+), somatostatin(+), and neuropeptide Y(+)) interneurons (Cobos et al., 2005).
Figures

References
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997a;19:27–37. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997b;278:474–476. - PubMed
-
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. - PMC - PubMed
-
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. - PubMed
-
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
