A bihormonal closed-loop artificial pancreas for type 1 diabetes
- PMID: 20393188
- PMCID: PMC4242106
- DOI: 10.1126/scitranslmed.3000619
A bihormonal closed-loop artificial pancreas for type 1 diabetes
Abstract
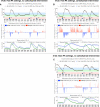
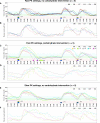
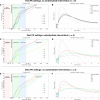
Automated control of blood glucose (BG) concentration is a long-sought goal for type 1 diabetes therapy. We have developed a closed-loop control system that uses frequent measurements of BG concentration along with subcutaneous delivery of both the fast-acting insulin analog lispro and glucagon (to imitate normal physiology) as directed by a computer algorithm. The algorithm responded only to BG concentrations and incorporated a pharmacokinetic model for lispro. Eleven subjects with type 1 diabetes and no endogenous insulin secretion were studied in 27-hour experiments, which included three carbohydrate-rich meals. In six subjects, the closed-loop system achieved a mean BG concentration of 140 mg/dl, which is below the mean BG concentration target of < or =154 mg/dl recommended by the American Diabetes Association. There were no instances of treatment-requiring hypoglycemia. Five other subjects exhibited hypoglycemia that required treatment; however, these individuals had slower lispro absorption kinetics than the six subjects that did not become hypoglycemic. The time-to-peak plasma lispro concentrations of subjects that exhibited hypoglycemia ranged from 71 to 191 min (mean, 117 +/- 48 min) versus 56 to 72 min (mean, 64 +/- 6 min) in the group that did not become hypoglycemic (aggregate mean of 84 min versus 31 min longer than the algorithm's assumption of 33 min, P = 0.07). In an additional set of experiments, adjustment of the algorithm's pharmacokinetic parameters (time-to-peak plasma lispro concentration set to 65 min) prevented hypoglycemia in both groups while achieving an aggregate mean BG concentration of 164 mg/dl. These results demonstrate the feasibility of safe BG control by a bihormonal artificial endocrine pancreas.
Figures
Comment in
-
Optimal control of blood glucose: the diabetic patient or the machine?Sci Transl Med. 2010 Apr 14;2(27):27ps18. doi: 10.1126/scitranslmed.3001083. Sci Transl Med. 2010. PMID: 20393187 Free PMC article.
References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

