Population differences in platinum toxicity as a means to identify novel genetic susceptibility variants
- PMID: 20393316
- PMCID: PMC2908307
- DOI: 10.1097/FPC.0b013e3283396c4e
Population differences in platinum toxicity as a means to identify novel genetic susceptibility variants
Abstract
Objectives: Clinical studies show that Asians (ASN) are more susceptible to toxicities associated with platinum-containing regimens. We hypothesized that studying ASN as an 'enriched phenotype' population could enable the discovery of novel genetic determinants of platinum susceptibility.
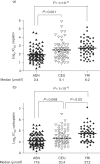
Methods: Using well-genotyped lymphoblastoid cell lines from the HapMap, we determined cisplatin and carboplatin cytotoxicity phenotypes (IC50s) for ASN, Caucasians (CEU), and Africans (YRI). IC50s were used in genome-wide association studies.
Results: ASN were most sensitive to platinums, corroborating clinical findings. ASN genome-wide association studies produced 479 single-nucleotide polymorphisms (SNPs) associating with cisplatin susceptibility and 199 with carboplatin susceptibility (P<10). Considering only the most significant variants (P<9.99x10), backwards elimination was then used to identify reduced-model SNPs, which robustly described the drug phenotypes within ASN. These SNPs comprised highly descriptive genetic signatures of susceptibility, with 12 SNPs explaining more than 95% of the susceptibility phenotype variation for cisplatin, and eight SNPs approximately 75% for carboplatin. To determine the possible function of these variants in ASN, the SNPs were tested for association with differential expression of target genes. SNPs were highly associated with the expression of multiple target genes, and notably, the histone H3 family was implicated for both drugs, suggesting a platinum-class mechanism. Histone H3 has repeatedly been described as regulating the formation of platinum-DNA adducts, but this is the first evidence that specific genetic variants might mediate these interactions in a pharmacogenetic manner. Finally, to determine whether any ASN-identified SNPs might also be important in other human populations, we interrogated all 479/199 SNPs for association with platinum susceptibility in an independent combined CEU/YRI population. Three unique SNPs for cisplatin and 10 for carboplatin replicated in CEU/YRI.
Conclusion: Enriched 'platinum susceptible' populations can be used to discover novel genetic determinants governing interindividual platinum chemotherapy susceptibility.
Figures



Similar articles
-
Integration of genetic and functional genomics data to uncover chemotherapeutic induced cytotoxicity.Pharmacogenomics J. 2019 Apr;19(2):178-190. doi: 10.1038/s41397-018-0024-6. Epub 2018 May 25. Pharmacogenomics J. 2019. PMID: 29795408
-
Genetic variation that predicts platinum sensitivity reveals the role of miR-193b* in chemotherapeutic susceptibility.Mol Cancer Ther. 2012 Sep;11(9):2054-61. doi: 10.1158/1535-7163.MCT-12-0221. Epub 2012 Jun 29. Mol Cancer Ther. 2012. PMID: 22752226 Free PMC article.
-
Chemotherapeutic-induced apoptosis: a phenotype for pharmacogenomics studies.Pharmacogenet Genomics. 2011 Aug;21(8):476-88. doi: 10.1097/FPC.0b013e3283481967. Pharmacogenet Genomics. 2011. PMID: 21642893 Free PMC article.
-
Pharmacogenomics for the efficacy of platinum-based chemotherapy: Old drugs, new integrated perspective.Biomed Pharmacother. 2020 Jun;126:110057. doi: 10.1016/j.biopha.2020.110057. Epub 2020 Mar 4. Biomed Pharmacother. 2020. PMID: 32145590 Review.
-
Platinum resistance: laboratory findings and clinical implications.Stem Cells. 1993 May;11(3):182-93. doi: 10.1002/stem.5530110304. Stem Cells. 1993. PMID: 8318904 Review.
Cited by
-
Extending the lymphoblastoid cell line model for drug combination pharmacogenomics.Pharmacogenomics. 2021 Jun;22(9):543-551. doi: 10.2217/pgs-2020-0160. Epub 2021 May 28. Pharmacogenomics. 2021. PMID: 34044623 Free PMC article. Review.
-
Review of genetic and pharmacogenetic differences in cytotoxic and targeted therapies for pancreatic cancer in African Americans.J Natl Med Assoc. 2023 Apr;115(2):164-174. doi: 10.1016/j.jnma.2023.01.008. Epub 2023 Feb 17. J Natl Med Assoc. 2023. PMID: 36801148 Free PMC article. Review.
-
Functional consequences of PRPF39 on distant genes and cisplatin sensitivity.Hum Mol Genet. 2012 Oct 1;21(19):4348-55. doi: 10.1093/hmg/dds266. Epub 2012 Jul 5. Hum Mol Genet. 2012. PMID: 22773733 Free PMC article.
-
Clinical Evaluation of Cisplatin Sensitivity of Germline Polymorphisms in Neoadjuvant Chemotherapy for Urothelial Cancer.Clin Genitourin Cancer. 2016 Dec;14(6):511-517. doi: 10.1016/j.clgc.2016.03.006. Epub 2016 Mar 10. Clin Genitourin Cancer. 2016. PMID: 27150640 Free PMC article.
-
Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer.Biomed Res Int. 2015;2015:413076. doi: 10.1155/2015/413076. Epub 2015 Jun 7. Biomed Res Int. 2015. PMID: 26137480 Free PMC article. Review.
References
-
- Celik I, Kars A, Ozyar E, Tekuzman G, Atahan L, Firat D. Major toxicity of cisplatin, fluorouracil, and leucovorin following chemoradiotherapy in patients with nasopharyngeal carcinoma [comment] J Clin Oncol. 1996;14:1043–1044. - PubMed
-
- Van Glabbeke M, Renard J, Pinedo HM, Cavalli F, Vermorken J, Sessa C, et al. Iproplatin and carboplatin induced toxicities: overview of phase II clinical trial conducted by the EORTC Early Clinical Trials Cooperative Group (ECTG) Eur J Cancer Clin Oncol. 1988;24:255–262. - PubMed
-
- Dolan ME, Newbold KG, Nagasubramanian R, Wu X, Ratain MJ, Cook EH, Jr, Badner JA. Heritability and linkage analysis of sensitivity to cisplatin-induced cytotoxicity. Cancer Res. 2004;64:4353–4356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

