Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo
- PMID: 20393650
- PMCID: PMC2946841
- DOI: 10.1039/c000237b
Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo
Abstract
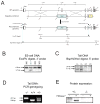
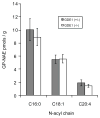
The biosynthesis of the endocannabinoid anandamide (AEA) and related N-acyl ethanolamine (NAE) lipids is complex and appears to involve multiple pathways, including: (1) direct release of NAEs from N-acyl phosphatidyl ethanolamine (NAPE) precursors by the phosphodiesterase NAPE-PLD, and (2) double O-deacylation of NAPEs followed by phosphodiester bond hydrolysis of the resulting glycero-phospho (GP)-NAEs. We recently identified GDE1 as a GP-NAE phosphodiesterase that may be involved in the second pathway. Here, we report the generation and characterization of GDE1(-/-) mice, which are viable and overtly normal in their cage behavior. Brain homogenates from GDE1(-/-) mice exhibit a near-complete loss of detectable GP-NAE phosphodiesterase activity; however, bulk brain levels of AEA and other NAEs were unaltered in these animals. To address the possibility of compensatory pathways, we generated GDE1(-/-)/NAPE-PLD(-/-) mice. Conversion of NAPE to NAE was virtually undetectable in brain homogenates from these animals as measured under standard assay conditions, but again, bulk changes in brain NAEs were not observed. Interestingly, significant reductions in the accumulation of brain NAEs, including anandamide, were detected in GDE1(-/-)/NAPE-PLD(-/-) mice treated with a fatty acid amide hydrolase (FAAH) inhibitor that blocks NAE degradation. Finally, we determined that primary neurons from GDE1(-/-)/NAPE-PLD(-/-) mice can convert NAPEs to NAEs by a pathway that is not preserved following cell homogenization. In summary, combined inactivation of GDE1 and NAPE-PLD results in partial disruption of NAE biosynthesis, while also pointing to the existence of an additional enzymatic pathway(s) that converts NAPEs to NAEs. Characterization of this pathway should provide clarity on the multifaceted nature of NAE biosynthesis.
Figures
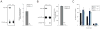
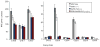
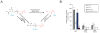
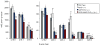
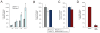
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

