Biosynthesis of unusual moth pheromone components involves two different pathways in the navel orangeworm, Amyelois transitella
- PMID: 20393784
- PMCID: PMC2866370
- DOI: 10.1007/s10886-010-9777-3
Biosynthesis of unusual moth pheromone components involves two different pathways in the navel orangeworm, Amyelois transitella
Abstract
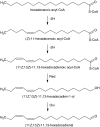
The sex pheromone of the navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae), consists of two different types of components, one type including (11Z,13Z)-11,13-hexadecadienal (11Z,13Z-16:Ald) with a terminal functional group containing oxygen, similar to the majority of moth pheromones reported, and another type including the unusual long-chain pentaenes, (3Z,6Z,9Z,12Z,15Z)-3,6,9,12,15-tricosapentaene (3Z,6Z,9Z,12Z,15Z-23:H) and (3Z,6Z,9Z,12Z,15Z)- 3,6,9,12,15-pentacosapentaene (3Z,6Z,9Z,12Z,15Z-25:H). After decapitation of females, the titer of 11Z,13Z-16:Ald in the pheromone gland decreased significantly, whereas the titer of the pentaenes remained unchanged. Injection of a pheromone biosynthesis activating peptide (PBAN) into the abdomens of decapitated females restored the titer of 11Z,13Z-16:Ald and even increased it above that in intact females, whereas the titer of the pentaenes in the pheromone gland was not affected by PBAN injection. In addition to common fatty acids, two likely precursors of 11Z,13Z-16:Ald, i.e., (Z)-11-hexadecenoic and (11Z,13Z)-11,13-hexadecadienoic acid, as well as traces of (Z)-6-hexadecenoic acid, were found in gland extracts. In addition, pheromone gland lipids contained (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoic acid, which also was found in extracts of the rest of the abdomen. Deuterium-labeled fatty acids, (16,16,16-D(3))-hexadecanoic acid and (Z)-[13,13,14,14,15,15,16,16,16-D(9)]-11-hexadecenoic acid, were incorporated into 11Z,13Z-16:Ald after topical application to the sex pheromone gland coupled with abdominal injection of PBAN. Deuterium label was incorporated into the C(23) and C(25) pentaenes after injection of (9Z,12Z,15Z)- [17,17,18,18,18-D(5)]-9,12,15-octadecatrienoic acid into 1-2 d old female pupae. These labeling results, in conjunction with the composition of fatty acid intermediates found in pheromone gland extracts, support different pathways leading to the two pheromone components. 11Z,13Z-16:Ald is probably produced in the pheromone gland by Delta11 desaturation of palmitic acid to 11Z-16:Acid followed by a second desaturation to form 11Z,13Z-16:Acid and subsequent reduction and oxidation. The production of 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H may take place outside the pheromone gland, and appears to start from linolenic acid, which is elongated and desaturated to form (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoic acid, followed by two or three further elongation steps and finally reductive decarboxylation.
Figures






Similar articles
-
Identification of critical secondary components of the sex pheromone of the navel orangeworm (Lepidoptera: Pyralidae).J Econ Entomol. 2010 Apr;103(2):314-30. doi: 10.1603/ec09177. J Econ Entomol. 2010. PMID: 20429444
-
Biosynthesis and PBAN-regulated transport of pheromone polyenes in the winter moth, Operophtera brumata.J Chem Ecol. 2013 Jun;39(6):790-6. doi: 10.1007/s10886-013-0292-1. Epub 2013 May 12. J Chem Ecol. 2013. PMID: 23665955
-
Biosynthesis of (3Z,6Z,9Z)-3,6,9-octadecatriene: the main component of the pheromone blend of Erannis bajaria.J Chem Ecol. 2007 Aug;33(8):1505-9. doi: 10.1007/s10886-007-9324-z. Epub 2007 Jul 4. J Chem Ecol. 2007. PMID: 17610118
-
Pheromone biosynthesis activating neuropeptide: from discovery to current status.Arch Insect Biochem Physiol. 1993;22(1-2):141-51. doi: 10.1002/arch.940220112. Arch Insect Biochem Physiol. 1993. PMID: 8431595 Review.
-
The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor.Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Prog Lipid Res. 2013. PMID: 24056189 Free PMC article. Review.
Cited by
-
Linoleic acid and stearic acid are biosynthetic precursors of (7Z,10Z)-7,10-hexadecadienal, the major component of the sex pheromone of Chilecomadia valdiviana (Lepidoptera: Cossidae).PLoS One. 2019 Apr 23;14(4):e0215769. doi: 10.1371/journal.pone.0215769. eCollection 2019. PLoS One. 2019. PMID: 31013309 Free PMC article.
-
Antennal transcriptome analysis of the piercing moth Oraesia emarginata (Lepidoptera: Noctuidae).PLoS One. 2017 Jun 14;12(6):e0179433. doi: 10.1371/journal.pone.0179433. eCollection 2017. PLoS One. 2017. PMID: 28614384 Free PMC article.
-
Identification of the Female-Produced Sex Pheromone of the Leafminer Holocacista capensis Infesting Grapevine in South Africa.J Chem Ecol. 2015 Aug;41(8):724-31. doi: 10.1007/s10886-015-0611-9. Epub 2015 Aug 14. J Chem Ecol. 2015. PMID: 26271672 Free PMC article.
-
A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids.Nutrients. 2018 Nov 4;10(11):1662. doi: 10.3390/nu10111662. Nutrients. 2018. PMID: 30400360 Free PMC article. Review.
-
Sex Pheromones of Two Leafminer Species, Antispila oinophylla and Holocacista rivillei (Lepidoptera: Heliozelidae) Infesting Grapevine in Italy.J Chem Ecol. 2019 Jan;45(1):1-8. doi: 10.1007/s10886-018-1036-z. Epub 2018 Dec 14. J Chem Ecol. 2019. PMID: 30547362 Free PMC article.
References
-
- ANDO T, OHTANI K, YAMAMOTO M, MIYAMOTO T, QIN XR. Sex pheromone of Japanese giant looper, Ascotis selenaria cretacea: identification and field tests. J. Chem. Ecol. 1997;23:2413–2423. doi: 10.1023/B:JOEC.0000006683.58028.1e. - DOI
-
- ANDO T, Inomata SI, Yamamoto M. Lepidopteran sex pheromones. In: Schulz S, editor. The Chemistry of Pheromones and Other Semiochemicals I: Topics in Current Chemistry. Berlin, Heidelberg, New York: Springer; 2004. pp. 51–96. - PubMed
-
- Ando T, Kawai T, Matsuoka K. Epoxyalkenyl sex pheromones produced by female moths in highly evolved groups: biosynthesis and its endocrine regulation. J. Pestic. Sci. 2008;33:17–20. doi: 10.1584/jpestics.R07-06. - DOI
-
- Bjostad LB, Roelofs WL. Sex pheromone biosynthetic precursors in Bombyx mori. Insect Biochem. 1984;14:275–278. doi: 10.1016/0020-1790(84)90060-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

