Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach
- PMID: 20394060
- PMCID: PMC3198871
- DOI: 10.1002/cne.22323
Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach
Abstract
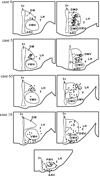
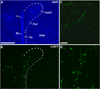
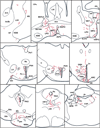
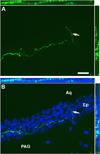
Tracing the axonal projections of selected neurons is labor intensive and inherently limited by currently available neuroanatomical methods. We developed an adeno-associated virus (AAV) that can be used for efficiently tracing identified neuronal populations. The virus encodes a humanized Renilla green fluorescent protein (hrGFP) that is transcriptionally silenced by a neo cassette flanked by LoxH/LoxP sites (AAV-lox-Stop-hrGFP). Thus, hrGFP is expressed only in neurons with Cre recombinase activity. To demonstrate the utility of this approach, the virus was injected unilaterally into the dorsomedial hypothalamus (DMH) of mice that express Cre in neurons expressing the leptin receptor. Animals with DMH injections showed robust hrGFP expression in DMH neurons, as visualized by its endogenous fluorescence or following immunolabeling. We found that hrGFP was expressed in approximately one-third to one-half of Cre-expressing neurons at the site of injection, but not in non-Cre-expressing neurons. The expression of GFP allowed us to identify the projection fields of DMH leptin-responsive neurons. Our results show hrGFP-positive axonal projections and terminals in the paraventricular nucleus of the hypothalamus, arcuate nucleus, preoptic area, bed nucleus of the stria terminalis, paraventricular thalamus, periaqueductal gray, and precoeruleus. The aforementioned pattern of projections was similar to DMH projections determined by injections of biotinylated dextran amine in the mouse DMH. Interestingly, some hrGFP-positive terminals were seen contacting the ependymal layer of the third and fourth ventricles. In summary, this approach is an effective tool for tracing axonal projections of chemically identified neurons, including leptin-responsive neurons.
(c) 2010 Wiley-Liss, Inc.
Figures








Similar articles
-
Cholinergic neurons in the dorsomedial hypothalamus regulate food intake.Mol Metab. 2017 Jan 12;6(3):306-312. doi: 10.1016/j.molmet.2017.01.001. eCollection 2017 Mar. Mol Metab. 2017. PMID: 28271037 Free PMC article.
-
Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice.J Neurosci. 2004 Mar 17;24(11):2797-805. doi: 10.1523/JNEUROSCI.5369-03.2004. J Neurosci. 2004. PMID: 15028773 Free PMC article.
-
Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse.Cells Tissues Organs. 2009;190(5):256-85. doi: 10.1159/000209233. Epub 2009 Mar 13. Cells Tissues Organs. 2009. PMID: 19287129
-
A fourth generation of neuroanatomical tracing techniques: exploiting the offspring of genetic engineering.J Neurosci Methods. 2014 Sep 30;235:331-48. doi: 10.1016/j.jneumeth.2014.07.021. Epub 2014 Aug 11. J Neurosci Methods. 2014. PMID: 25107853 Review.
-
Genetic identification of preoptic neurons that regulate body temperature in mice.Temperature (Austin). 2022 Jan 9;9(1):14-22. doi: 10.1080/23328940.2021.1993734. eCollection 2022. Temperature (Austin). 2022. PMID: 35655663 Free PMC article. Review.
Cited by
-
Cholinergic neurons in the mouse rostral ventrolateral medulla target sensory afferent areas.Brain Struct Funct. 2013 Mar;218(2):455-75. doi: 10.1007/s00429-012-0408-3. Epub 2012 Mar 30. Brain Struct Funct. 2013. PMID: 22460939 Free PMC article.
-
Molecular anatomy of the gut-brain axis revealed with transgenic technologies: implications in metabolic research.Front Neurosci. 2013 Jul 31;7:134. doi: 10.3389/fnins.2013.00134. eCollection 2013. Front Neurosci. 2013. PMID: 23914153 Free PMC article.
-
Low-dose infusions of leptin into the nucleus of the solitary tract increase sensitivity to third ventricle leptin.Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E719-E728. doi: 10.1152/ajpendo.00562.2018. Epub 2019 Feb 5. Am J Physiol Endocrinol Metab. 2019. PMID: 30721096 Free PMC article.
-
How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake.Temperature (Austin). 2015 Oct 12;2(3):406-17. doi: 10.1080/23328940.2015.1075095. eCollection 2015 Jul-Sep. Temperature (Austin). 2015. PMID: 27227054 Free PMC article. Review.
-
Cell-Type Identification in the Autonomic Nervous System.Neurosci Bull. 2019 Feb;35(1):145-155. doi: 10.1007/s12264-018-0284-9. Epub 2018 Aug 31. Neurosci Bull. 2019. PMID: 30171526 Free PMC article. Review.
References
-
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27(7):1763–1770. - PubMed
-
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431–442. - PubMed
-
- Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1(4):619–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH61583/MH/NIMH NIH HHS/United States
- R01 DK088423/DK/NIDDK NIH HHS/United States
- 1UL1RR024923/RR/NCRR NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- R01 DK053301/DK/NIDDK NIH HHS/United States
- DK53301/DK/NIDDK NIH HHS/United States
- NS33987/NS/NINDS NIH HHS/United States
- 1PL1DK081182/DK/NIDDK NIH HHS/United States
- R01 MH061583/MH/NIMH NIH HHS/United States
- R01 NS033987/NS/NINDS NIH HHS/United States
- TL1 DK081181/DK/NIDDK NIH HHS/United States
- UL1 RR024923/RR/NCRR NIH HHS/United States
- PL1 DK081182/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

