Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity
- PMID: 20394071
- PMCID: PMC3435885
- DOI: 10.1002/smll.201000028
Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity
Abstract
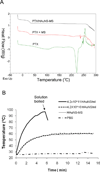
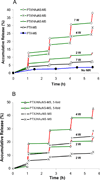
Despite advances in controlled drug delivery, reliable methods for activatable, high-resolution control of drug release are needed. The hypothesis that the photothermal effect mediated by a near-infrared (NIR) laser and hollow gold nanospheres (HAuNSs) could modulate the release of anticancer agents is tested with biodegradable and biocompatible microspheres (1-15 microm) containing the antitumor drug paclitaxel (PTX) and HAuNSs (approximately 35 nm in diameter), which display surface plasmon absorbance in the NIR region. HAuNS-containing microspheres exhibit a NIR-induced thermal effect similar to that of plain HAuNSs. Rapid, repetitive PTX release from the PTX/HAuNS-containing microspheres is observed upon irradiation with NIR light (808 nm), whereas PTX release is insignificant when the NIR light is switched off. The release of PTX from the microspheres is readily controlled by the output power of the NIR laser, duration of irradiation, treatment frequency, and concentration of HAuNSs embedded inside the microspheres. In vitro, cancer cells incubated with PTX/HAuNS-loaded microspheres and irradiated with NIR light display significantly greater cytotoxic effects than cells incubated with the microspheres alone or cells irradiated with NIR light alone, owing to NIR-light-triggered drug release. Treatment of human U87 gliomas and MDA-MB-231 mammary tumor xenografts in nude mice with intratumoral injections of PTX/HAuNS-loaded microspheres followed by NIR irradiation results in significant tumor-growth delay compared to tumors treated with HAuNS-loaded microspheres (no PTX) and NIR irradiation or with PTX/HAuNS-loaded microspheres alone. The data support the feasibility of a therapeutic approach in which NIR light is used for simultaneous modulation of drug release and induction of photothermal cell killing.
Figures





References
-
- Perelman LA, Pacholski C, Li YY, VanNieuwenhze MS, Sailor MJ. Nanomed. 2008;3:31. - PubMed
-
- Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. J. Am. Chem. Soc. 2003;125:4451. - PubMed
-
- Meers P. Adv. Drug Deliver. Rev. 2001;53:265. - PubMed
-
- Ankareddi I, Hampel ML, Sewell MK, Kim D-H. Nanotech. 2007;2:431.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

