Revealing noncovalent interactions
- PMID: 20394428
- PMCID: PMC2864795
- DOI: 10.1021/ja100936w
Revealing noncovalent interactions
Abstract
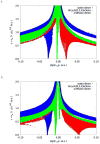
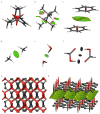
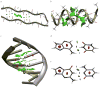
Molecular structure does not easily identify the intricate noncovalent interactions that govern many areas of biology and chemistry, including design of new materials and drugs. We develop an approach to detect noncovalent interactions in real space, based on the electron density and its derivatives. Our approach reveals the underlying chemistry that compliments the covalent structure. It provides a rich representation of van der Waals interactions, hydrogen bonds, and steric repulsion in small molecules, molecular complexes, and solids. Most importantly, the method, requiring only knowledge of the atomic coordinates, is efficient and applicable to large systems, such as proteins or DNA. Across these applications, a view of nonbonded interactions emerges as continuous surfaces rather than close contacts between atom pairs, offering rich insight into the design of new and improved ligands.
Figures

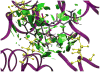
References
-
- Fenniri H, Packiarajan M, Vidale KL, Sherman DM, Hallenga K, Wood KV, Stowell JG. J Am Chem Soc. 2001;123:3854–3855. - PubMed
-
- Kruse P, Johnson ER, DiLabio GA, Wolkow RA. Nano Lett. 2002;2:807–810.
-
- Sheiko SS, Sun FC, Randall A, Shirvanyants D, Rubinstein M, Lee H, Matyjaszewski K. Nature. 2006;440:191–194. - PubMed
-
- DiLabio GA, Piva PG, Kruse P, Wolkow RA. J Am Chem Soc. 2004;126:16048–16050. - PubMed
-
- Kollman PA. Chem Rev. 1977;10:365–371.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

