Assignment of the structure of petrocortyne A by mixture syntheses of four candidate stereoisomers
- PMID: 20394446
- PMCID: PMC2871111
- DOI: 10.1021/jo100115h
Assignment of the structure of petrocortyne A by mixture syntheses of four candidate stereoisomers
Abstract
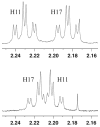
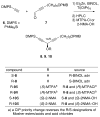
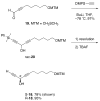
Two different mixture synthesis routes have been used to make the four stereoisomers of petrocortyne A. A first quick and dirty route provided a mixture of the four isomers in nonselective fashion. Mosher and 2-naphthylmethoxyacetic acid (NMA) ester methods were developed to identify the components, and the mixture was partially resolved on analytical chiral HPLC to give the two pure enantiomers of petrocortyne A and the racemate of its diastereomer. A second fluorous mixture synthesis produced all four isomers of petrocortyne A in individual pure form. Comparison of spectra of Mosher derivatives of the synthetic isomers with two supposedly different natural products showed that both natural samples were instead identical and had the (3S,14S) configuration. Likewise, petrocortynes B, D, and F-H are (3S,14S) and petrocortyne D is (3R,14S). Having access to all possible candidate isomers of both petrocortyne A and its Mosher derivatives provided a secure structure assignment not so much because one of the isomers matched the natural product, but because all of the other isomers did not.
Figures
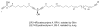

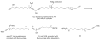

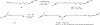

Similar articles
-
A "shortcut" Mosher ester method to assign configurations of stereocenters in nearly symmetric environments. Fluorous mixture synthesis and structure assignment of petrocortyne A.J Am Chem Soc. 2009 Apr 22;131(15):5411-3. doi: 10.1021/ja900849f. J Am Chem Soc. 2009. PMID: 19323551 Free PMC article.
-
On the proof and disproof of natural product stereostructures: characterization and analysis of a twenty-eight member stereoisomer library of murisolins and their Mosher ester derivatives.J Am Chem Soc. 2006 Aug 2;128(30):9943-56. doi: 10.1021/ja062469l. J Am Chem Soc. 2006. PMID: 16866554
-
Total synthesis and absolute configuration of (-)-gummiferol.Org Lett. 2011 Jul 15;13(14):3644-7. doi: 10.1021/ol201301b. Epub 2011 Jun 21. Org Lett. 2011. PMID: 21688861
-
[Esters and stereoisomers].Anaesthesist. 1997 Apr;46(4):282-6. doi: 10.1007/s001010050402. Anaesthesist. 1997. PMID: 9229981 Review. German.
-
Stereoisomers of cannabidiols and their pharmacological activities - A potentially novel direction for cannabinoids.Bioorg Med Chem. 2025 Jan 1;117:118019. doi: 10.1016/j.bmc.2024.118019. Epub 2024 Nov 23. Bioorg Med Chem. 2025. PMID: 39612769 Review.
Cited by
-
Marine bioactives: pharmacological properties and potential applications against inflammatory diseases.Mar Drugs. 2012 Apr;10(4):812-833. doi: 10.3390/md10040812. Epub 2012 Apr 5. Mar Drugs. 2012. PMID: 22690145 Free PMC article. Review.
-
Minimal fluorous tagging strategy that enables the synthesis of the complete stereoisomer library of SCH725674 macrolactones.J Am Chem Soc. 2012 May 9;134(18):7963-70. doi: 10.1021/ja302260d. Epub 2012 May 1. J Am Chem Soc. 2012. PMID: 22515682 Free PMC article.
-
Asymmetric catalytic synthesis of the proposed structure of trocheliophorolide B.Org Lett. 2012 Sep 7;14(17):4698-700. doi: 10.1021/ol302074h. Epub 2012 Aug 22. Org Lett. 2012. PMID: 22913543 Free PMC article.
References
-
- Shin J, Seo Y, Cho KW. J Nat Prod. 1998;61:1268–1273. - PubMed
- Lim YJ, Kim JS, Im KS, Jung JH, Lee CO, Hong J, Kim DK. J Nat Prod. 1999;62:1215–1217. - PubMed
- Lim YJ, Lee CO, Hong J, Kim DK, Im KS, Jung JH. J Nat Prod. 2001;64:1565–1567. - PubMed
- Lim YJ, Park HS, Im KS, Lee C-O, Hong J, Lee M-Y, Kim D-k, Jung JH. J Nat Prod. 2001;64:46–53. - PubMed
-
- Kim DK, Lee MY, Lee HS, Lee DS, Lee JR, Lee BJ, Jung JH. Cancer Lett. 2002;185:95–101. - PubMed
-
- Seo Y, Cho KW, Rho JR, Shin J, Sim CJ. Tetrahedron. 1998;54:447–462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

