High-throughput expression and purification of membrane proteins
- PMID: 20394823
- PMCID: PMC2933282
- DOI: 10.1016/j.jsb.2010.03.021
High-throughput expression and purification of membrane proteins
Abstract
High-throughput (HT) methodologies have had a tremendous impact on structural biology of soluble proteins. High-resolution structure determination relies on the ability of the macromolecule to form ordered crystals that diffract X-rays. While crystallization remains somewhat empirical, for a given protein, success is proportional to the number of conditions screened and to the number of variants trialed. HT techniques have greatly increased the number of targets that can be trialed and the rate at which these can be produced. In terms of number of structures solved, membrane proteins appear to be lagging many years behind their soluble counterparts. Likewise, HT methodologies for production and characterization of these hydrophobic macromolecules are only now emerging. Presented here is an HT platform designed exclusively for membrane proteins that has processed over 5000 targets.
Figures




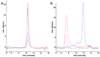
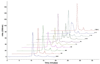
References
-
- Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nat Struct Biol. 2003;10:980. - PubMed
-
- Henderson R, Unwin PN. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975;257:28–32. - PubMed
-
- Deisenhofer J, Epp O, Miki K, Huber R, Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984;180:385–398. - PubMed
-
- Grisshammer R, Tate CG. Overexpression of integral membrane proteins for structural studies. Q Rev Biophys. 1995;28:315–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

