Ectopic expression of X-linked lymphocyte-regulated protein pM1 renders tumor cells resistant to antitumor immunity
- PMID: 20395201
- PMCID: PMC2861497
- DOI: 10.1158/0008-5472.CAN-09-3856
Ectopic expression of X-linked lymphocyte-regulated protein pM1 renders tumor cells resistant to antitumor immunity
Abstract
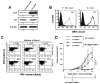
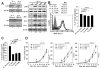
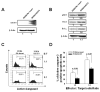
Tumor immune escape is a major obstacle in cancer immunotherapy, but the mechanisms involved remain poorly understood. We have previously developed an immune evasion tumor model using an in vivo immune selection strategy and revealed Akt-mediated immune resistance to antitumor immunity induced by various cancer immunotherapeutic agents. In the current study, we used microarray gene analysis to identify an Akt-activating candidate molecule overexpressed in immune-resistant tumors compared with parental tumors. X-linked lymphocyte-regulated protein pM1 (XLR) gene was the most upregulated in immune-resistant tumors compared with parental tumor cells. Furthermore, the retroviral transduction of XLR in parental tumor cells led to activation of Akt, resulting in upregulation of antiapoptotic proteins and the induction of immune resistance phenotype in parental tumor cells. In addition, we found that transduction of parental tumor cells with other homologous genes from the mouse XLR family, such as synaptonemal complex protein 3 (SCP3) and XLR-related, meiosis-regulated protein (XMR) and its human counterpart of SCP3 (hSCP3), also led to activation of Akt, resulting in the upregulation of antiapoptotic proteins and induction of immune resistance phenotype. Importantly, characterization of a panel of human cervical cancers revealed relatively higher expression levels of hSCP3 in human cervical cancer tissue compared with normal cervical tissue. Thus, our data indicate that ectopic expression of XLR and its homologues in tumor cells represents a potentially important mechanism for tumor immune evasion and serves as a promising molecular target for cancer immunotherapy.
(c) 2010 AACR.
Figures



References
-
- Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

