Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer
- PMID: 20395368
- PMCID: PMC2854397
- DOI: 10.1101/gad.1897010
Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer
Abstract
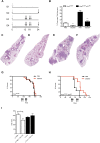
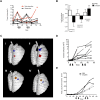
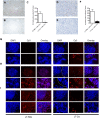
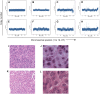
Chemotherapy resistance is a major obstacle in cancer treatment, yet the mechanisms of response to specific therapies have been largely unexplored in vivo. Employing genetic, genomic, and imaging approaches, we examined the dynamics of response to a mainstay chemotherapeutic, cisplatin, in multiple mouse models of human non-small-cell lung cancer (NSCLC). We show that lung tumors initially respond to cisplatin by sensing DNA damage, undergoing cell cycle arrest, and inducing apoptosis-leading to a significant reduction in tumor burden. Importantly, we demonstrate that this response does not depend on the tumor suppressor p53 or its transcriptional target, p21. Prolonged cisplatin treatment promotes the emergence of resistant tumors with enhanced repair capacity that are cross-resistant to platinum analogs, exhibit advanced histopathology, and possess an increased frequency of genomic alterations. Cisplatin-resistant tumors express elevated levels of multiple DNA damage repair and cell cycle arrest-related genes, including p53-inducible protein with a death domain (Pidd). We demonstrate a novel role for PIDD as a regulator of chemotherapy response in human lung tumor cells.
Figures

Similar articles
-
FEN1 promotes tumor progression and confers cisplatin resistance in non-small-cell lung cancer.Mol Oncol. 2017 Jun;11(6):640-654. doi: 10.1002/1878-0261.12058. Epub 2017 May 12. Mol Oncol. 2017. PMID: 28371273 Free PMC article.
-
Cisplatin resistance in non-small cell lung cancer cells is associated with an abrogation of cisplatin-induced G2/M cell cycle arrest.PLoS One. 2017 Jul 26;12(7):e0181081. doi: 10.1371/journal.pone.0181081. eCollection 2017. PLoS One. 2017. PMID: 28746345 Free PMC article.
-
Simultaneous targeting of ATM and Mcl-1 increases cisplatin sensitivity of cisplatin-resistant non-small cell lung cancer.Cancer Biol Ther. 2017 Aug 3;18(8):606-615. doi: 10.1080/15384047.2017.1345391. Epub 2017 Jul 7. Cancer Biol Ther. 2017. PMID: 28686074 Free PMC article.
-
[Advances of DNA damage repair and Cisplatin resistance mechanisms in lung cancer].Zhongguo Fei Ai Za Zhi. 2011 Dec;14(12):960-4. doi: 10.3779/j.issn.1009-3419.2011.12.11. Zhongguo Fei Ai Za Zhi. 2011. PMID: 22152698 Free PMC article. Review. Chinese.
-
The role of DNA repair pathways in cisplatin resistant lung cancer.Cancer Treat Rev. 2014 Dec;40(10):1161-70. doi: 10.1016/j.ctrv.2014.10.003. Epub 2014 Oct 18. Cancer Treat Rev. 2014. PMID: 25458603 Review.
Cited by
-
Preclinical Murine Models for Lung Cancer: Clinical Trial Applications.Biomed Res Int. 2015;2015:621324. doi: 10.1155/2015/621324. Epub 2015 May 3. Biomed Res Int. 2015. PMID: 26064932 Free PMC article. Review.
-
Screening of the siGPCR library in combination with cisplatin against lung cancers.Sci Rep. 2022 Oct 17;12(1):17358. doi: 10.1038/s41598-022-21063-0. Sci Rep. 2022. PMID: 36253428 Free PMC article.
-
Response to Different Oxygen Partial Pressures and Evolution Analysis of Apoptosis-Related Genes in Plateau Zokor (Myospalax baileyi).Front Genet. 2022 Jun 8;13:865301. doi: 10.3389/fgene.2022.865301. eCollection 2022. Front Genet. 2022. PMID: 35754836 Free PMC article.
-
Cross-Resistance Among Sequential Cancer Therapeutics: An Emerging Issue.Front Oncol. 2022 Jun 23;12:877380. doi: 10.3389/fonc.2022.877380. eCollection 2022. Front Oncol. 2022. PMID: 35814399 Free PMC article. Review.
-
Growth pattern analysis of murine lung neoplasms by advanced semi-automated quantification of micro-CT images.PLoS One. 2013 Dec 23;8(12):e83806. doi: 10.1371/journal.pone.0083806. eCollection 2013. PLoS One. 2013. PMID: 24376755 Free PMC article.
References
-
- Ahrendt SA, Chow JT, Yang SC, Wu L, Zhang MJ, Jen J, Sidransky D 2000. Alcohol consumption and cigarette smoking increase the frequency of p53 mutations in non-small cell lung cancer. Cancer Res 60: 3155–3159 - PubMed
-
- American Cancer Society 2007. American Cancer Society Cancer Facts and Figures 2007. American Cancer Society, Atlanta, GA
-
- Bando T, Fujimura M, Kasahara K, Matsuda T 1998. Significance of Na+, K+-ATPase on intracellular accumulation of cis-diamminedichloroplatinum(II) in human non-small-cell but not in small-cell lung cancer cell lines. Anticancer Res 18: 1085–1089 - PubMed
-
- Blommaert FA, van Dijk-Knijnenburg HC, Dijt FJ, den Engelse L, Baan RA, Berends F, Fichtinger-Schepman AM 1995. Formation of DNA adducts by the anticancer drug carboplatin: Different nucleotide sequence preferences in vitro and in cells. Biochemistry 34: 8474–8480 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
