PKC-delta promotes renal tubular cell apoptosis associated with proteinuria
- PMID: 20395372
- PMCID: PMC3152238
- DOI: 10.1681/ASN.2009070760
PKC-delta promotes renal tubular cell apoptosis associated with proteinuria
Abstract
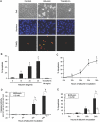
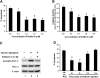
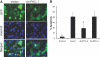
Proteinuria may contribute to progressive renal damage by inducing tubulointerstitial inflammation, fibrosis, and tubular cell injury and death, but the mechanisms underlying these pathologic changes remain largely unknown. Here, in a rat kidney proximal tubular cell line (RPTC), albumin induced apoptosis in a time- and dose-dependent manner. Caspase activation accompanied albumin-induced apoptosis, and general caspase inhibitors could suppress this activation. In addition, Bcl-2 transfection inhibited apoptosis and attenuated albumin-induced Bax translocation to mitochondria and cytochrome c release from the organelles, further confirming a role for the intrinsic pathway of apoptosis in albuminuria-associated tubular apoptosis. We observed phosphorylation and activation of PKC-delta early during treatment of RPTC cells with albumin. Rottlerin, a pharmacologic inhibitor of PKC-delta, suppressed albumin-induced Bax translocation, cytochrome c release, and apoptosis. Moreover, a dominant-negative mutant of PKC-delta blocked albumin-induced apoptosis in RPTC cells. In vivo, we observed activated PKC-delta in proteinuric kidneys of streptozotocin-induced diabetic mice and in kidneys after direct albumin overload. Notably, albumin overload induced apoptosis in renal tubules, which was less severe in PKC-delta-knockout mice. Taken together, these results suggest that activation of PKC-delta promotes tubular cell injury and death during albuminuria, broadening our understanding of the pathogenesis of progressive proteinuric kidney diseases.
Figures


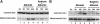

Comment in
-
Is PKC-delta a new killer molecule in kidney?J Am Soc Nephrol. 2010 Jul;21(7):1063-5. doi: 10.1681/ASN.2010050528. Epub 2010 Jun 17. J Am Soc Nephrol. 2010. PMID: 20558537 No abstract available.
References
-
- Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 - PubMed
-
- Perico N, Benigni A, Remuzzi G: Present and future drug treatments for chronic kidney diseases: Evolving targets in renoprotection. Nat Rev Drug Discov 7: 936–953, 2008 - PubMed
-
- Strutz FM: EMT and proteinuria as progression factors. Kidney Int 75: 475–481, 2009 - PubMed
-
- Chang SS: Albuminuria and diabetic nephropathy. Pediatr Endocrinol Rev 5[Suppl 4]: 974–979, 2008 - PubMed
-
- Erkan E, De Leon M, Devarajan P: Albumin overload induces apoptosis in LLC-PK(1) cells. Am J Physiol Renal Physiol 280: F1107–F1114, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

