Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing
- PMID: 20395417
- PMCID: PMC3324225
- DOI: 10.1182/blood-2009-11-254334
Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing
Abstract
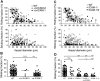
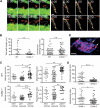
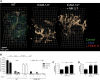
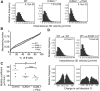
Although it is well established that stromal intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and vascular cell adhesion molecule-1 (VCAM-1) mediate lymphocyte recruitment into peripheral lymph nodes (PLNs), their precise contributions to the individual steps of the lymphocyte homing cascade are not known. Here, we provide in vivo evidence for a selective function for ICAM-1 > ICAM-2 > VCAM-1 in lymphocyte arrest within noninflamed PLN microvessels. Blocking all 3 CAMs completely inhibited lymphocyte adhesion within PLN high endothelial venules (HEVs). Post-arrest extravasation of T cells was a 3-step process, with optional ICAM-1-dependent intraluminal crawling followed by rapid ICAM-1- or ICAM-2-independent diapedesis and perivascular trapping. Parenchymal motility of lymphocytes was modestly reduced in the absence of ICAM-1, while ICAM-2 and alpha4-integrin ligands were not required for B-cell motility within follicles. Our findings highlight nonredundant functions for stromal Ig family CAMs in shear-resistant lymphocyte adhesion in steady-state HEVs, a unique role for ICAM-1 in intraluminal lymphocyte crawling but redundant roles for ICAM-1 and ICAM-2 in lymphocyte diapedesis and interstitial motility.
Figures


References
-
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8(10):764–775. - PubMed
-
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005:23127–159. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. - PubMed
-
- Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181(9):6189–6200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

