Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression
- PMID: 20395535
- PMCID: PMC2904108
- DOI: 10.1152/ajpgi.00522.2010
Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression
Abstract
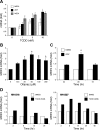
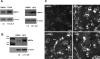
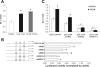
Multidrug resistance protein 4 (MRP4; ABCC4) is an ATP binding cassette transporter that facilitates the excretion of bile salt conjugates and other conjugated steroids in hepatocytes and renal proximal tubule epithelium. MRP4/Mrp4 undergoes adaptive upregulation in response to oxidative and cholestatic liver injury in human and animal models of cholestasis. However, the molecular mechanism of this regulation remains to be determined. The aryl hydrocarbon receptor (AhR) and NF-E2-related factor 2 (Nrf2) play important roles in protecting cells from oxidative stress. Here we examine the role of these two nuclear factors in the regulation of the expression of human MRP4. HepG2 cells and human hepatocytes were treated with the AhR and Nrf2 activators, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 3-methylcholanthrene (3-MC), or oltipraz and other nuclear receptor agonists. TCDD, 3-MC, and oltipraz significantly increased MRP4 expression at mRNA and protein levels. Computer program analysis revealed three Xenobiotic response element (XRE) and one Maf response element sites within the first 500 bp of the MRP4 proximal promoter. Luciferase reporter assay detected strong promoter activity (53-fold higher than vector control) in this region. TCDD and 3-MC also induced promoter activity in the reporter assays. Mutation of any of these XRE sites significantly decreased MRP4 promoter activity in reporter assays, although XRE2 demonstrated the strongest effects on both basal and TCDD-inducible activity. EMSA and chromatin immunoprecipitation assays further confirmed that both AhR and Nrf2 bind to the proximal promoter of MRP4. Our findings indicate that AhR and Nrf2 play important roles in regulating MRP4 expression and suggest that agents that activate their activity may be of therapeutic benefit for cholestasis.
Figures
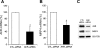


Similar articles
-
Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines.Toxicol Appl Pharmacol. 2014 Feb 1;274(3):408-16. doi: 10.1016/j.taap.2013.12.002. Epub 2013 Dec 16. Toxicol Appl Pharmacol. 2014. PMID: 24355420 Free PMC article.
-
Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes.Drug Metab Dispos. 2006 Oct;34(10):1756-63. doi: 10.1124/dmd.106.010033. Epub 2006 Jul 12. Drug Metab Dispos. 2006. PMID: 16837569
-
Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression.Hepatology. 2009 Nov;50(5):1588-96. doi: 10.1002/hep.23151. Hepatology. 2009. PMID: 19821532 Free PMC article.
-
The AHR-NRF2-JDP2 gene battery: Ligand-induced AHR transcriptional activation.Biochem Pharmacol. 2025 Mar;233:116761. doi: 10.1016/j.bcp.2025.116761. Epub 2025 Jan 23. Biochem Pharmacol. 2025. PMID: 39855429 Review.
-
Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors.Mutat Res. 2004 Nov 2;555(1-2):149-71. doi: 10.1016/j.mrfmmm.2004.05.023. Mutat Res. 2004. PMID: 15476858 Review.
Cited by
-
Nrf2 signaling increases expression of ATP-binding cassette subfamily C mRNA transcripts at the blood-brain barrier following hypoxia-reoxygenation stress.Fluids Barriers CNS. 2017 Mar 16;14(1):6. doi: 10.1186/s12987-017-0055-4. Fluids Barriers CNS. 2017. PMID: 28298215 Free PMC article.
-
The antiandrogen flutamide is a novel aryl hydrocarbon receptor ligand that disrupts bile acid homeostasis in mice through induction of Abcc4.Biochem Pharmacol. 2016 Nov 1;119:93-104. doi: 10.1016/j.bcp.2016.08.021. Epub 2016 Aug 26. Biochem Pharmacol. 2016. PMID: 27569425 Free PMC article.
-
Protein Abundance of Drug Transporters in Human Hepatitis C Livers.Int J Mol Sci. 2022 Jul 19;23(14):7947. doi: 10.3390/ijms23147947. Int J Mol Sci. 2022. PMID: 35887291 Free PMC article.
-
Multiple drug resistance-associated protein 4 (MRP4), prostaglandin transporter (PGT), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) as determinants of PGE2 levels in cancer.Prostaglandins Other Lipid Mediat. 2015 Jan-Mar;116-117:99-103. doi: 10.1016/j.prostaglandins.2014.11.003. Epub 2014 Nov 27. Prostaglandins Other Lipid Mediat. 2015. PMID: 25433169 Free PMC article. Review.
-
Characterization of arsenic hepatobiliary transport using sandwich-cultured human hepatocytes.Toxicol Sci. 2015 Jun;145(2):307-20. doi: 10.1093/toxsci/kfv051. Epub 2015 Mar 9. Toxicol Sci. 2015. PMID: 25752797 Free PMC article.
References
-
- Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones 11: 356–363, 2006 - PMC - PubMed
-
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279: 22250–22257, 2004 - PubMed
-
- Auyeung DJ, Kessler FK, Ritter JK. Mechanism of rat UDP-glucuronosyltransferase 1A6 induction by oltipraz: evidence for a contribution of the Aryl hydrocarbon receptor pathway. Mol Pharmacol 63: 119–127, 2003 - PubMed
-
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol 68: 160–168, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

