Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart
- PMID: 20395592
- PMCID: PMC2895561
- DOI: 10.1161/CIRCRESAHA.109.214353
Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart
Abstract
Rationale: Erythropoietin (EPO) is often administered to cardiac patients with anemia, particularly from chronic kidney disease, and stimulation of erythropoiesis may stabilize left ventricular and renal function by recruiting protective effects beyond the correction of anemia.
Objective: We examined the hypothesis that EPO receptor (EpoR) ligand-binding, which activates endothelial NO synthase (eNOS), regulates the prosurvival program of mitochondrial biogenesis in the heart.
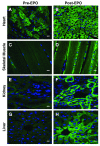
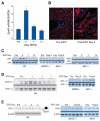
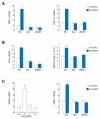
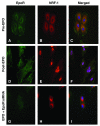
Methods and results: We investigated the effects of EPO on mitochondrial biogenesis over 14 days in healthy mice. Mice expressing a mitochondrial green fluorescent protein reporter construct demonstrated sharp increases in myocardial mitochondrial density after 3 days of EPO administration that peaked at 7 days and surpassed hepatic or renal effects and anteceded significant increases in blood hemoglobin content. Quantitatively, in wild-type mice, complex II activity, state 3 respiration, and mtDNA copy number increased significantly; also, resting energy expenditure and natural running speed improved, with no evidence of an increase in left ventricular mass index. Mechanistically, EPO activated cardiac mitochondrial biogenesis by enhancement of nuclear respiratory factor-1, PGC-1alpha (peroxisome proliferator-activated receptor gamma coactivator 1alpha), and mitochondrial transcription factor-A gene expression in wild-type but not in eNOS(-/-) or protein kinase B (Akt1)(-/-) mice. EpoR was required, because EpoR silencing in cardiomyocytes blocked EPO-mediated nuclear translocation of nuclear respiratory factor-1.
Conclusions: These findings support a new physiological and protective role for EPO, acting through its cell surface receptor and eNOS-Akt1 signal transduction, in matching cardiac mitochondrial mass to the convective O(2) transport capacity as erythrocyte mass expands.
Figures

References
-
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

