The primary cilium: a signalling centre during vertebrate development
- PMID: 20395968
- PMCID: PMC3121168
- DOI: 10.1038/nrg2774
The primary cilium: a signalling centre during vertebrate development
Abstract
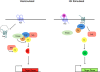
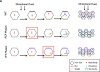
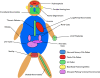
The primary cilium has recently stepped into the spotlight, as a flood of data show that this organelle has crucial roles in vertebrate development and human genetic diseases. Cilia are required for the response to developmental signals, and evidence is accumulating that the primary cilium is specialized for hedgehog signal transduction. The formation of cilia, in turn, is regulated by other signalling pathways, possibly including the planar cell polarity pathway. The cilium therefore represents a nexus for signalling pathways during development. The connections between cilia and developmental signalling have begun to clarify the basis of human diseases associated with ciliary dysfunction.
Figures





References
-
- Pedersen LB, Veland IR, Schroder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. - PubMed
-
- Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. - PubMed
-
- Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

