Chemical blocking of zinc ions in CNS increases neuronal damage following traumatic brain injury (TBI) in mice
- PMID: 20396380
- PMCID: PMC2852423
- DOI: 10.1371/journal.pone.0010131
Chemical blocking of zinc ions in CNS increases neuronal damage following traumatic brain injury (TBI) in mice
Abstract
Background: Traumatic brain injury (TBI) is one of the leading causes of disability and death among young people. Although much is already known about secondary brain damage the full range of brain tissue responses to TBI remains to be elucidated. A population of neurons located in cerebral areas associated with higher cognitive functions harbours a vesicular zinc pool co-localized with glutamate. This zinc enriched pool of synaptic vesicles has been hypothesized to take part in the injurious signalling cascade that follows pathological conditions such as seizures, ischemia and traumatic brain injury. Pathological release of excess zinc ions from pre-synaptic vesicles has been suggested to mediate cell damage/death to postsynaptic neurons.
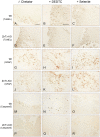
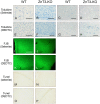
Methodology/principal findings: In order to substantiate the influence of vesicular zinc ions on TBI, we designed a study in which damage and zinc movements were analysed in several different ways. Twenty-four hours after TBI ZnT3-KO mice (mice without vesicular zinc) were compared to littermate Wild Type (WT) mice (mice with vesicular zinc) with regard to histopathology. Furthermore, in order to evaluate a possible neuro-protective dimension of chemical blocking of vesicular zinc, we treated lesioned mice with either DEDTC or selenite. Our study revealed that chemical blocking of vesicular zinc ions, either by chelation with DEDTC or accumulation in zinc-selenium nanocrystals, worsened the effects on the aftermath of TBI in the WT mice by increasing the number of necrotic and apoptotic cells within the first 24 hours after TBI, when compared to those of chemically untreated WT mice.
Conclusion/significance: ZnT3-KO mice revealed more damage after TBI compared to WT controls. Following treatment with DEDTC or selenium an increase in the number of both dead and apoptotic cells were seen in the controls within the first 24 hours after TBI while the degree of damage in the ZnT3-KO mice remained largely unchanged. Further analyses revealed that the damage development in the two mouse strains was almost identical after either zinc chelation or zinc complexion therapy.
Conflict of interest statement
Figures

Similar articles
-
Changes in the vesicular zinc pattern following traumatic brain injury.Neuroscience. 2007 Nov 30;150(1):93-103. doi: 10.1016/j.neuroscience.2007.09.066. Epub 2007 Oct 9. Neuroscience. 2007. PMID: 17996379
-
Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats.J Cereb Blood Flow Metab. 2006 Feb;26(2):161-9. doi: 10.1038/sj.jcbfm.9600176. J Cereb Blood Flow Metab. 2006. PMID: 15988476
-
Alcohol dependence treating agent, acamprosate, prevents traumatic brain injury-induced neuron death through vesicular zinc depletion.Transl Res. 2019 May;207:1-18. doi: 10.1016/j.trsl.2019.01.002. Epub 2019 Jan 17. Transl Res. 2019. PMID: 30731068
-
Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function.Neurosci Biobehav Rev. 2017 Sep;80:329-350. doi: 10.1016/j.neubiorev.2017.06.006. Epub 2017 Jun 15. Neurosci Biobehav Rev. 2017. PMID: 28624432 Review.
-
The Protective Role of Glutathione on Zinc-Induced Neuron Death after Brain Injuries.Int J Mol Sci. 2023 Feb 2;24(3):2950. doi: 10.3390/ijms24032950. Int J Mol Sci. 2023. PMID: 36769273 Free PMC article. Review.
Cited by
-
CNS and CNS diseases in relation to their immune system.Front Immunol. 2022 Nov 16;13:1063928. doi: 10.3389/fimmu.2022.1063928. eCollection 2022. Front Immunol. 2022. PMID: 36466889 Free PMC article. Review.
-
Zinc in the central nervous system: From molecules to behavior.Biofactors. 2012 May-Jun;38(3):186-93. doi: 10.1002/biof.1012. Epub 2012 Mar 31. Biofactors. 2012. PMID: 22473811 Free PMC article. Review.
-
A Compact Blast-Induced Traumatic Brain Injury Model in Mice.J Neuropathol Exp Neurol. 2016 Feb;75(2):183-96. doi: 10.1093/jnen/nlv019. J Neuropathol Exp Neurol. 2016. PMID: 26802177 Free PMC article.
-
Total and Free Zinc Dynamics as Biomarkers for Neurological Impairment in Traumatic Spinal Cord Injury.Nutrients. 2025 Jan 29;17(3):496. doi: 10.3390/nu17030496. Nutrients. 2025. PMID: 39940353 Free PMC article.
-
Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury.Nutr Rev. 2012 Jul;70(7):410-3. doi: 10.1111/j.1753-4887.2012.00486.x. Epub 2012 May 22. Nutr Rev. 2012. PMID: 22747843 Free PMC article. Review.
References
-
- Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Rev. 2000;34(3):137–148. - PubMed
-
- Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003;85(3):563–570. - PubMed
-
- Pérez-Clausell J, Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985;337(1):91–98. - PubMed
-
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130(5S) Suppl:1471S–1483S. - PubMed
-
- Takeda A. Zinc homeostasis and functions of zinc in the brain. Biometals. 2001;14(3–4):343–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

