Influence of estrogen and polyamines on mifepristone-induced apoptosis in prostate cancer cells
- PMID: 20396571
- PMCID: PMC2855108
- DOI: 10.4143/crt.2004.36.1.85
Influence of estrogen and polyamines on mifepristone-induced apoptosis in prostate cancer cells
Abstract
Purpose: Although androgens are the main steroids controlling the growth of prostate glands, estrogens are also important in the regulation of its growth. Prostate cancer cells, like other cancer cells, maintain high levels of polyamines. In LNCaP cells, apoptosis is induced by mifepristone. During the process of cell death, the regulation of ROS production, caspase-3 activation and poly (ADP-ribose) polymerase cleavage were investigated in the presence of estrogen and polyamines to identify their possible roles.
Materials and methods: The cell growth was assessed using the MTT assay, and the intracellular ROS production by the DCFH-DA assay. The p53 protein expression, activation of caspase-3 and PARP cleavage were checked by Western blotting, with specific antibodies to each.
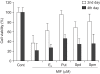
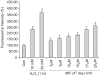
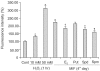
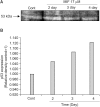
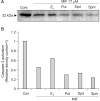
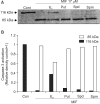
Results: The growth and viability of the cells were significantly inhibited, in a dose- and time-dependent manners, by mifepristone (MIF) treatment. The production of ROS were dependent on the MIF dosage. The activation of caspase-3 and cleavage of PARPalso increased with the duration of MIF treatment. The expression of p53 protein also increased with increases in the MIF incubation time. E(2) severely inhibited the ROS production, caspase-3 activation and PARP cleavage. However, polyamines only inhibited the ROS production, without influencing the caspase-3 activation or PARP cleavage.
Conclusion: In LNCaP cells, MIF induces apoptosis through ROS production. The expression of p53 protein, caspase-3 activation and PARP cleavage accompanied the process of apoptosis. The apoptotic processes were inhibited by E(2), but polyamines only inhibited the ROS production, implying the multifunctional role of E(2), in addition to its role as a free radical scavenger.
Keywords: Apoptosis; Estrogen; Mifepristone; Polyamine; Prostate cancer cell.
Figures
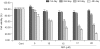
References
-
- El Etreby MF, Liang Y, Lewis RW. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. Prostate. 2000;43:31–42. - PubMed
-
- Eid MA, Lewis RW, Kumar MV. Mifepristone pretreatment overcomes resistance of prostate cancer cells to tumor necrosis factor α-related apoptosis-induced ligand. Mol Cancer Ther. 2002;1:831–840. - PubMed
-
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. - PMC - PubMed
-
- Nakamura T, Sakamoto K. Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem Biophys Res Commun. 2000;284:203–210. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

