Bone marrow derivation of interstitial cells of cajal in small intestine following intestinal injury
- PMID: 20396598
- PMCID: PMC2854535
- DOI: 10.1155/2010/164986
Bone marrow derivation of interstitial cells of cajal in small intestine following intestinal injury
Abstract
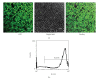
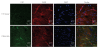
Interstitial cells of Cajal (ICCs) in gastrointestinal tract are specialized cells serving as pacemaker cells. The origin of ICCs is currently not fully characterized. In this work, we aimed to study whether bone marrow-derived cells (BMDCs) could contribute to the origin of ICCs in the muscular plexus of small intestine using GFP-C57BL/6 chimeric mice.Engraftment of BMDCs in the intestine was investigated for GFP expression. GFP positive bone marrow mononuclear cells reached a proportion of 95.65% +/- 3.72% at different times in chimerism. Donor-derived cells distributed widely in all the layers of the gastrointestinal tract. There were GFP positive BMDCs in the myenteric plexus, which resembled characteristics of ICCs, including myenteric location, c-Kit positive staining, and ramified morphology. Donor-derived ICCs in the myenteric plexus contributed to a percentage ranging 9.25% +/- 4.9% of all the ICCs in the myenteric plexus. In conclusion, here we described that donor-derived BMDCs might differentiate into gastrointestinal ICCs after radiation injury, which provided an alternative source for the origin of the ICCs in the muscular plexus of adult intestine. These results further identified the plasticity of BMDCs and indicated therapeutic implications of BMDCs for the gastrointestinal dysmotility caused by ICCs disorders.
Figures



References
-
- Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterology and Motility. 2008;20(supplement 1):54–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

