Asymmetric Reduction of Activated Alkenes by Pentaerythritol Tetranitrate Reductase: Specificity and Control of Stereochemical Outcome by Reaction Optimisation
- PMID: 20396613
- PMCID: PMC2854813
- DOI: 10.1002/adsc.200900603
Asymmetric Reduction of Activated Alkenes by Pentaerythritol Tetranitrate Reductase: Specificity and Control of Stereochemical Outcome by Reaction Optimisation
Abstract
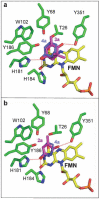
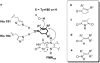
We show that pentaerythritol tetranitrate reductase (PETNR), a member of the 'ene' reductase old yellow enzyme family, catalyses the asymmetric reduction of a variety of industrially relevant activated alpha,beta-unsaturated alkenes including enones, enals, maleimides and nitroalkenes. We have rationalised the broad substrate specificity and stereochemical outcome of these reductions by reference to molecular models of enzyme-substrate complexes based on the crystal complex of the PETNR with 2-cyclohexenone 4a. The optical purity of products is variable (49-99% ee), depending on the substrate type and nature of substituents. Generally, high enantioselectivity was observed for reaction products with stereogenic centres at Cbeta (>99% ee). However, for the substrates existing in two isomeric forms (e.g., citral 11a or nitroalkenes 18-19a), an enantiodivergent course of the reduction of E/Z-forms may lead to lower enantiopurities of the products. We also demonstrate that the poor optical purity obtained for products with stereogenic centres at Calpha is due to non-enzymatic racemisation. In reactions with ketoisophorone 3a we show that product racemisation is prevented through reaction optimisation, specifically by shortening reaction time and through control of solution pH. We suggest this as a general strategy for improved recovery of optically pure products with other biocatalytic conversions where there is potential for product racemisation.
Figures


References
-
- Chaparro-Riggers JF, Rogers TA, Vazquez-Figueroa E, Polizzi KM, Bommarius AS. Adv. Synth. Catal. 2007;349:1521–1531.
-
- Hall M, Stueckler C, Ehammer H, Pointner E, Oberdorfer G, Gruber K, Hauer B, Stuermer R, Kroutil W, Macheroux P, Faber K. Adv. Synth. Catal. 2008;350:411–418.
-
- Hall M, Stueckler C, Hauer B, Stuermer R, Friedrich T, Breuer M, Kroutil W, Faber K. Eur. J. Org. Chem. 2008:1511–1516.
-
- Padhi SK, Bougioukou DJ, Stewart JD. J. Am. Chem. Soc. 2009;131:3271–3280. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
