HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities
- PMID: 20396973
- PMCID: PMC2914283
- DOI: 10.1007/s11481-010-9205-z
HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities
Abstract
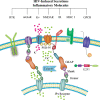
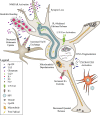
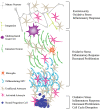
Human immunodeficiency virus type 1 (HIV) infection presently affects more that 40 million people worldwide, and is associated with central nervous system (CNS) disruption in at least 30% of infected individuals. The use of highly active antiretroviral therapy has lessened the incidence, but not the prevalence of mild impairment of higher cognitive and cortical functions (HIV-associated neurocognitive disorders) as well as substantially reduced a more severe form dementia (HIV-associated dementia). Furthermore, improving neurological outcomes will require novel, adjunctive therapies that are targeted towards mechanisms of HIV-induced neurodegeneration. Identifying such molecular and pharmacological targets requires an understanding of the events preceding irreversible neuronal damage in the CNS, such as actions of neurotoxins (HIV proteins and cellular factors), disruption of ion channel properties, synaptic damage, and loss of adult neurogenesis. By considering the specific mechanisms and consequences of HIV neuropathogenesis, unified approaches for neuroprotection will likely emerge using a tailored, combined, and non-invasive approach.
Figures
References
-
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. - PubMed
-
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–293. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

