Rebound discharge in deep cerebellar nuclear neurons in vitro
- PMID: 20396983
- PMCID: PMC2949560
- DOI: 10.1007/s12311-010-0168-7
Rebound discharge in deep cerebellar nuclear neurons in vitro
Abstract
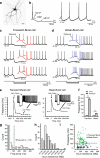
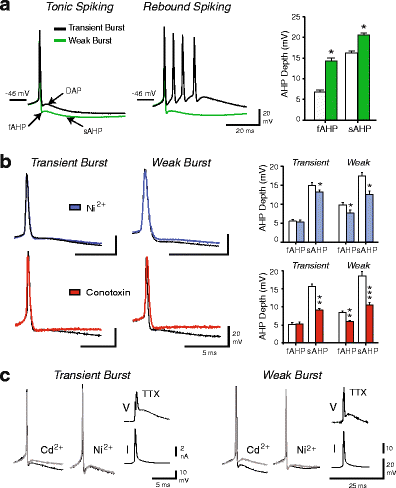
Neurons of the deep cerebellar nuclei (DCN) play a critical role in defining the output of cerebellum in the course of encoding Purkinje cell inhibitory inputs. The earliest work performed with in vitro preparations established that DCN cells have the capacity to translate membrane hyperpolarizations into a rebound increase in firing frequency. The primary means of distinguishing between DCN neurons has been according to cell size and transmitter phenotype, but in some cases, differences in the firing properties of DCN cells maintained in vitro have been reported. In particular, it was shown that large diameter cells in the rat DCN exhibit two phenotypes of rebound discharge in vitro that may eventually help define their functional roles in cerebellar output. A transient burst and weak burst phenotype can be distinguished based on the frequency and pattern of rebound discharge immediately following a hyperpolarizing stimulus. Work to date indicates that the difference in excitability arises from at least the degree of activation of T-type Ca(2+) current during the immediate phase of rebound firing and Ca(2+)-dependent K(+) channels that underlie afterhyperpolarizations. Both phenotypes can be detected following stimulation of Purkinje cell inhibitory inputs under conditions that preserve resting membrane potential and natural ionic gradients. In this paper, we review the evidence supporting the existence of different rebound phenotypes in DCN cells and the ion channel expression patterns that underlie their generation.
Figures


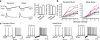

Similar articles
-
Determinants of rebound burst responses in rat cerebellar nuclear neurons to physiological stimuli.J Physiol. 2016 Feb 15;594(4):985-1003. doi: 10.1113/JP271894. Epub 2016 Jan 18. J Physiol. 2016. PMID: 26662168 Free PMC article.
-
Ionic factors governing rebound burst phenotype in rat deep cerebellar neurons.J Neurophysiol. 2008 Nov;100(5):2684-701. doi: 10.1152/jn.90427.2008. Epub 2008 Sep 3. J Neurophysiol. 2008. PMID: 18768644
-
Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons.Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5555-60. doi: 10.1073/pnas.0601261103. Epub 2006 Mar 27. Proc Natl Acad Sci U S A. 2006. PMID: 16567615 Free PMC article.
-
GABA-ergic transmission in deep cerebellar nuclei.Prog Neurobiol. 1997 Oct;53(2):259-71. doi: 10.1016/s0301-0082(97)00033-6. Prog Neurobiol. 1997. PMID: 9364613 Review.
-
Times of neuron origin and neurogenetic gradients in mice Purkinje cells and deep cerebellar nuclei neurons during the development of the cerebellum. A review.Tissue Cell. 2022 Oct;78:101897. doi: 10.1016/j.tice.2022.101897. Epub 2022 Aug 17. Tissue Cell. 2022. PMID: 35994919 Review.
Cited by
-
Evaluating the adaptive-filter model of the cerebellum.J Physiol. 2011 Jul 15;589(Pt 14):3459-70. doi: 10.1113/jphysiol.2010.201574. Epub 2011 Apr 18. J Physiol. 2011. PMID: 21502289 Free PMC article. Review.
-
Bidirectional modulation of deep cerebellar nuclear cells revealed by optogenetic manipulation of inhibitory inputs from Purkinje cells.Neuroscience. 2014 Sep 26;277:250-66. doi: 10.1016/j.neuroscience.2014.07.006. Epub 2014 Jul 11. Neuroscience. 2014. PMID: 25020121 Free PMC article.
-
Sex differences in cerebellar synaptic transmission and sex-specific responses to autism-linked Gabrb3 mutations in mice.Elife. 2016 Apr 14;5:e07596. doi: 10.7554/eLife.07596. Elife. 2016. PMID: 27077953 Free PMC article.
-
Cerebellar stimulation prevents Levodopa-induced dyskinesia in mice and normalizes activity in a motor network.Nat Commun. 2022 Jun 9;13(1):3211. doi: 10.1038/s41467-022-30844-0. Nat Commun. 2022. PMID: 35680891 Free PMC article.
-
Structural and functional brain changes in X-linked Charcot-Marie-Tooth disease: insights from a multimodal neuroimaging study.Neuroradiology. 2022 Mar;64(3):543-552. doi: 10.1007/s00234-021-02730-x. Epub 2021 Sep 9. Neuroradiology. 2022. PMID: 34498107
References
-
- Czubayko U, Sultan F, Thier P, Schwarz C. Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. J Neurophysiol. 2001;85(5):2017–2029. - PubMed
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82(4):1697–1709. - PubMed
-
- Uusisaari M, Obata K, Knopfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97(1):901–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

