Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts
- PMID: 20397300
- PMCID: PMC2925491
- DOI: 10.1111/j.1476-5381.2010.00665.x
Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts
Abstract
Background and purpose: Gallium (Ga) has been shown to be effective in the treatment of disorders associated with accelerated bone loss, including cancer-related hypercalcemia and Paget's disease. These clinical applications suggest that Ga could reduce bone resorption. However, few studies have studied the effects of Ga on osteoclastic resorption. Here, we have explored the effects of Ga on bone cells in vitro.
Experimental approach: In different osteoclastic models [osteoclasts isolated from long bones of neonatal rabbits (RBC), murine RAW 264.7 cells and human CD14-positive cells], we have performed resorption activity tests, staining for tartrate resistant acid phosphatase (TRAP), real-time polymerase chain reaction analysis, viability and apoptotic assays. We also evaluated the effect of Ga on osteoblasts in terms of proliferation, viability and activity by using an osteoblastic cell line (MC3T3-E1) and primary mouse osteoblasts.
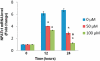
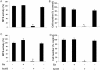
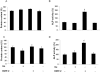
Key results: Gallium dose-dependently (0-100 microM) inhibited the in vitro resorption activity of RBC and induced a significant decrease in the expression level of transcripts coding for osteoclastic markers in RAW 264.7 cells. Ga also dramatically reduced the formation of TRAP-positive multinucleated cells. Ga down-regulated in a dose-dependant manner the expression of the transcription factor NFATc1. However, Ga did not affect the viability or activity of primary and MC3T3-E1 osteoblasts.
Conclusions and implications: Gallium exhibits a dose-dependent anti-osteoclastic effect by reducing in vitro osteoclastic resorption, differentiation and formation without negatively affecting osteoblasts. We provide evidence that this inhibitory mechanism involves down-regulation of NFATc1 expression, a master regulator of RANK-induced osteoclastic differentiation.
Figures



Similar articles
-
Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces.Clin Oral Implants Res. 2014 Jul;25(7):831-7. doi: 10.1111/clr.12146. Epub 2013 Apr 8. Clin Oral Implants Res. 2014. PMID: 23560589
-
Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts.J Cell Biochem. 2012 Jan;113(1):247-59. doi: 10.1002/jcb.23351. J Cell Biochem. 2012. PMID: 21898547
-
[Effect of the bone resorption supernatant from RAW264.7 osteoclast on the osteogenic activity of mouse MC3T3-E1 cell].Zhonghua Kou Qiang Yi Xue Za Zhi. 2012 Jan;47(1):32-7. doi: 10.3760/cma.j.issn.1002-0098.2012.01.012. Zhonghua Kou Qiang Yi Xue Za Zhi. 2012. PMID: 22490219 Chinese.
-
Alkaline phosphatase and tartrate-resistant acid phosphatase in osteoblasts of normal and pathologic bone.Ital J Anat Embryol. 2001;106(2 Suppl 1):129-33. Ital J Anat Embryol. 2001. PMID: 11729947 Review.
-
TNAP, TrAP, ecto-purinergic signaling, and bone remodeling.J Cell Biochem. 2008 Oct 15;105(3):655-62. doi: 10.1002/jcb.21885. J Cell Biochem. 2008. PMID: 18773425 Review.
Cited by
-
Osteogenic response and osteoprotective effects in vivo of a nanostructured titanium surface with antibacterial properties.J Mater Sci Mater Med. 2016 Mar;27(3):52. doi: 10.1007/s10856-015-5661-6. Epub 2016 Jan 19. J Mater Sci Mater Med. 2016. PMID: 26787484
-
Immunomodulatory biomaterials for implant-associated infections: from conventional to advanced therapeutic strategies.Biomater Res. 2022 Dec 5;26(1):72. doi: 10.1186/s40824-022-00326-x. Biomater Res. 2022. PMID: 36471454 Free PMC article. Review.
-
Can Spatiotemporal Fluoride (18F-) Uptake be Used to Assess Bone Formation in the Tibia? A Longitudinal Study Using PET/CT.Clin Orthop Relat Res. 2017 May;475(5):1486-1498. doi: 10.1007/s11999-017-5250-8. Epub 2017 Feb 1. Clin Orthop Relat Res. 2017. PMID: 28150226 Free PMC article.
-
Highly-Bioreactive Silica-Based Mesoporous Bioactive Glasses Enriched with Gallium(III).Materials (Basel). 2018 Mar 2;11(3):367. doi: 10.3390/ma11030367. Materials (Basel). 2018. PMID: 29498654 Free PMC article.
-
Na-doped β-tricalcium phosphate: physico-chemical and in vitro biological properties.J Mater Sci Mater Med. 2011 Mar;22(3):593-600. doi: 10.1007/s10856-010-4219-x. Epub 2011 Jan 8. J Mater Sci Mater Med. 2011. PMID: 21221733
References
-
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. - PubMed
-
- van Balen R, Steyerberg EW, Polder JJ, Ribbers TL, Habbema JD, Cools HJ. Hip fracture in elderly patients: outcomes for function, quality of life, and type of residence. Clin Orthop Relat Res. 2001;390:232–243. - PubMed
-
- Benezeth P, Diakonov II, Pokrovski GS, Dandurand JL, Schott J, Khodakovsky IL. Gallium speciation in aqueous solution. Experimental study and modelling.2. Solubility of alpha-GaOOH in acidic solutions from 150 to 250 degrees C and hydrolysis constants of gallium (III) to 300 degrees C. Geochimica Et Cosmochimica Acta. 1997;61:1345–1357.
-
- Blair HC. How the osteoclast degrades bone. Bioessays. 1998;20:837–846. - PubMed
-
- Blair HC, Teitelbaum SL, Tan HL, Schlesinger PH. Reversible inhibition of osteoclastic activity by bone-bound gallium (III) J Cell Biochem. 1992;48:401–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

