Alternatives to gadolinium-based metal chelates for magnetic resonance imaging
- PMID: 20397688
- PMCID: PMC2874212
- DOI: 10.1021/cr900284a
Alternatives to gadolinium-based metal chelates for magnetic resonance imaging
Figures


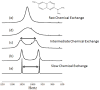












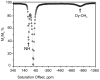

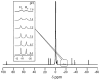
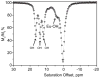
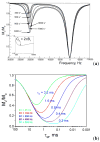

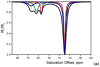




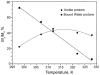

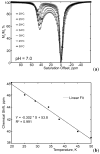





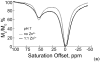



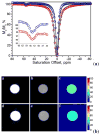
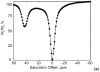








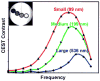

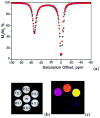
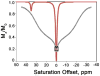
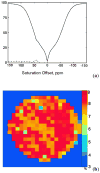
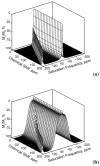
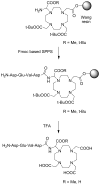
 ) at 315 K (■). CEST spectroscopy conditions (7 T): irradiation power = 4.5 μT. Reproduced with permission from reference , Copyright 2008 American Chemical Society.
) at 315 K (■). CEST spectroscopy conditions (7 T): irradiation power = 4.5 μT. Reproduced with permission from reference , Copyright 2008 American Chemical Society.




















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

