Solvation response along the reaction coordinate in the active site of ketosteroid isomerase
- PMID: 20397697
- PMCID: PMC2871671
- DOI: 10.1021/ja1007849
Solvation response along the reaction coordinate in the active site of ketosteroid isomerase
Abstract
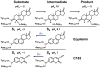
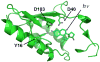
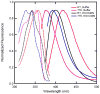
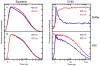
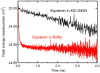
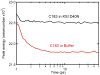
A light-activated reaction analog has been developed to mimic the catalytic reaction cycle of Delta(5)-3-ketosteroid isomerase to probe the functionally relevant protein solvation response to the catalytic charge transfer. Delta(5)-3-ketosteroid isomerase from Pseudomonas putida catalyzes a C-H bond cleavage and formation through an enolate intermediate. Conversion of the ketone substrate to the enolate intermediate is simulated by a photoacid bound to the active site oxyanion hole. In the ground state, the photoacid electrostatically resembles the enolate intermediate while the low pK(a) excited state resembles the ketone starting material. Time-resolved fluorescence experiments with photoacids coumarin 183 and equilenin show the active site of Delta(5)-3-ketosteroid isomerase to be largely unperturbed by the light-activated reaction. The small solvation response for the photoacid at the active site as compared with a simple solvent suggests the active site does not significantly change its electrostatic environment during the catalytic cycle. Instead, the reaction takes place in an electrostatically preorganized environment.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

