The essential neutral sphingomyelinase is involved in the trafficking of the variant surface glycoprotein in the bloodstream form of Trypanosoma brucei
- PMID: 20398210
- PMCID: PMC2904498
- DOI: 10.1111/j.1365-2958.2010.07151.x
The essential neutral sphingomyelinase is involved in the trafficking of the variant surface glycoprotein in the bloodstream form of Trypanosoma brucei
Abstract
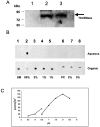
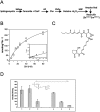
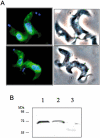
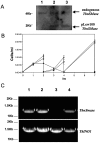
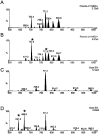
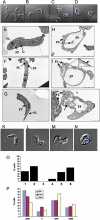
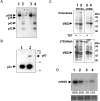
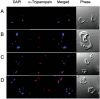
Sphingomyelin is the main sphingolipid in Trypanosoma brucei, the causative agent of African sleeping sickness. In vitro and in vivo characterization of the T. brucei neutral sphingomyelinase demonstrates that it is directly involved in sphingomyelin catabolism. Gene knockout studies in the bloodstream form of the parasite indicate that the neutral sphingomyelinase is essential for growth and survival, thus highlighting that the de novo biosynthesis of ceramide is unable to compensate for the loss of sphingomyelin catabolism. The phenotype of the conditional knockout has given new insights into the highly active endocytic and exocytic pathways in the bloodstream form of T. brucei. Hence, the formation of ceramide in the endoplasmic reticulum affects post-Golgi sorting and rate of deposition of newly synthesized GPI-anchored variant surface glycoprotein on the cell surface. This directly influences the corresponding rate of endocytosis, via the recycling endosomes, of pre-existing cell surface variant surface glycoprotein. The trypanosomes use this coupled endocytic and exocytic mechanism to maintain the cell density of its crucial variant surface glycoprotein protective coat. TbnSMase is therefore genetically validated as a drug target against African trypanosomes, and suggests that interfering with the endocytic transport of variant surface glycoprotein is a highly desirable strategy for drug development against African trypanosomasis.
Figures
Similar articles
-
Substrate specificity of the neutral sphingomyelinase from Trypanosoma brucei.Parasitology. 2019 Apr;146(5):604-616. doi: 10.1017/S0031182018001853. Epub 2018 Nov 5. Parasitology. 2019. PMID: 30392480 Free PMC article.
-
De novo sphingolipid synthesis is essential for viability, but not for transport of glycosylphosphatidylinositol-anchored proteins, in African trypanosomes.Eukaryot Cell. 2007 Mar;6(3):454-64. doi: 10.1128/EC.00283-06. Epub 2007 Jan 12. Eukaryot Cell. 2007. PMID: 17220466 Free PMC article.
-
Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei.J Cell Sci. 2004 Mar 1;117(Pt 7):1105-15. doi: 10.1242/jcs.00938. J Cell Sci. 2004. PMID: 14996937
-
The glycosylation of the variant surface glycoproteins and procyclic acidic repetitive proteins of Trypanosoma brucei.Mol Biochem Parasitol. 1998 Mar 1;91(1):145-52. doi: 10.1016/s0166-6851(97)00187-4. Mol Biochem Parasitol. 1998. PMID: 9574932 Review.
-
Life and times: synthesis, trafficking, and evolution of VSG.Trends Parasitol. 2014 May;30(5):251-8. doi: 10.1016/j.pt.2014.03.004. Epub 2014 Apr 12. Trends Parasitol. 2014. PMID: 24731931 Free PMC article. Review.
Cited by
-
The lipidome of Crithidia fasiculataand its plasticity.Front Cell Infect Microbiol. 2022 Oct 28;12:945750. doi: 10.3389/fcimb.2022.945750. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36405970 Free PMC article.
-
Sphingolipid and ceramide homeostasis: potential therapeutic targets.Biochem Res Int. 2012;2012:248135. doi: 10.1155/2012/248135. Epub 2012 Feb 9. Biochem Res Int. 2012. PMID: 22400113 Free PMC article.
-
Requirement for acetyl-CoA carboxylase in Trypanosoma brucei is dependent upon the growth environment.Mol Microbiol. 2011 Apr;80(1):117-32. doi: 10.1111/j.1365-2958.2011.07563.x. Epub 2011 Feb 16. Mol Microbiol. 2011. PMID: 21306439 Free PMC article.
-
Substrate specificity of the neutral sphingomyelinase from Trypanosoma brucei.Parasitology. 2019 Apr;146(5):604-616. doi: 10.1017/S0031182018001853. Epub 2018 Nov 5. Parasitology. 2019. PMID: 30392480 Free PMC article.
-
Exploring the activity of the putative Δ6-desaturase and its role in bloodstream form life-cycle transitions in Trypanosoma brucei.PLoS Pathog. 2025 Feb 18;21(2):e1012691. doi: 10.1371/journal.ppat.1012691. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39965027 Free PMC article.
References
-
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, et al. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem. 1999;264:545–553. - PubMed
-
- Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J Cell Sci. 2002;115:3255–3263. - PubMed
-
- Ali BRS, Pal A, Croft SL, Taylor RT, Field MC. The farnesyltransferase inhibitor manumycin A is a novel trypanocide with a complex mode of action including major effects on mitochondria. Mol Biochem Para. 1999;104:67–80. - PubMed
-
- Arenz C, Thutewohl M, Block O, Waldmann H, Altenbach HJ, Giannis A. Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chem Biochem. 2001;2:141–143. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

