Ion transport regulation by P2Y receptors, protein kinase C and phosphatidylinositol 3-kinase within the semicircular canal duct epithelium
- PMID: 20398257
- PMCID: PMC2862037
- DOI: 10.1186/1756-0500-3-100
Ion transport regulation by P2Y receptors, protein kinase C and phosphatidylinositol 3-kinase within the semicircular canal duct epithelium
Abstract
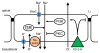
Background: The ionic composition of the luminal fluid in the vestibular labyrinth is maintained within tight limits by the many types of epithelial cells bounding the lumen. Regulatory mechanisms include systemic, paracrine and autocrine hormones along with their associated intracellular signal pathways. The epithelium lining the semicircular canal duct (SCCD) is a tissue that is known to absorb sodium and calcium and to secrete chloride.
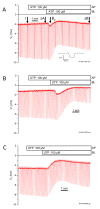
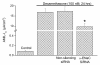
Findings: Transport function was assessed by measurements of short circuit current (Isc) and gene transcript expression was evaluated by microarray. Neither ATP nor UTP (100 microM) on the apical side of the epithelium had any effect on Isc. By contrast, basolateral ATP transiently increased Isc and transepithelial resistance dropped significantly after basolateral ATP and UTP. P2Y2 was the sole UTP-sensitive purinergic receptor expressed. Isc was reduced by 42%, 50% and 63% after knockdown of alpha-ENaC, stimulation of PKC and inhibition of PI3-K, while the latter two increased the transepithelial resistance. PKCdelta, PKCgamma and PI3-K were found to be expressed.
Conclusions: These observations demonstrate that ion transport by the SCCD is regulated by P2Y2 purinergic receptors on the basolateral membrane that may respond to systemic or local agonists, such as ATP and/or UTP. The sodium absorption from endolymph mediated by ENaC in SCCD is regulated by signal pathways that include the kinases PKC and PI3-K. These three newly-identified regulatory components may prove to be valuable drug targets in the control of pathologic vestibular conditions involving dysfunction of transport homeostasis in the ear, such as Meniere's disease.
Figures
Similar articles
-
Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat.Physiol Genomics. 2006 Jan 12;24(2):114-23. doi: 10.1152/physiolgenomics.00006.2005. Epub 2005 Nov 1. Physiol Genomics. 2006. PMID: 16263802
-
Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel.Am J Physiol Renal Physiol. 2004 Jun;286(6):F1127-35. doi: 10.1152/ajprenal.00387.2003. Epub 2004 Mar 2. Am J Physiol Renal Physiol. 2004. PMID: 14996671
-
Evidence for Purinergic Receptors in Vestibular Dark Cell and Strial Marginal Cell Epithelia of Gerbil.Audit Neurosci. 1995;1(4):331-340. Audit Neurosci. 1995. PMID: 22582019 Free PMC article.
-
Control of epithelial transport via luminal P2 receptors.Am J Physiol Renal Physiol. 2003 Mar;284(3):F419-32. doi: 10.1152/ajprenal.00075.2002. Am J Physiol Renal Physiol. 2003. PMID: 12556361 Review.
-
Vibrating probes: new technology for investigation of endolymph homeostasis.Keio J Med. 1996 Dec;45(4):301-5. doi: 10.2302/kjm.45.301. Keio J Med. 1996. PMID: 9023447 Review.
Cited by
-
Purinergic signaling in the peripheral vestibular system.Purinergic Signal. 2022 Jun;18(2):165-176. doi: 10.1007/s11302-022-09855-5. Epub 2022 Mar 28. Purinergic Signal. 2022. PMID: 35344126 Free PMC article. Review.
-
Regulation of sodium transport in the inner ear.Hear Res. 2011 Oct;280(1-2):21-9. doi: 10.1016/j.heares.2011.05.003. Epub 2011 May 18. Hear Res. 2011. PMID: 21620939 Free PMC article. Review.
-
cAMP-stimulated Cl- secretion is increased by glucocorticoids and inhibited by bumetanide in semicircular canal duct epithelium.BMC Physiol. 2013 Mar 27;13:6. doi: 10.1186/1472-6793-13-6. BMC Physiol. 2013. PMID: 23537040 Free PMC article.
-
Sodium selectivity of semicircular canal duct epithelial cells.BMC Res Notes. 2011 Sep 13;4:355. doi: 10.1186/1756-0500-4-355. BMC Res Notes. 2011. PMID: 21914199 Free PMC article.
References
-
- Marcus DC, Wangemann P. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System--From molecules to diseases. Alvarez-Leefmans FJ, Delpire E, editor. Elsevier; 2009. Cochlear and Vestibular Function and Dysfunction; pp. 421–433.
-
- Milhaud PG, Pondugula SR, Lee JH, Herzog M, Lehouelleur J, Wangemann P, Sans A, Marcus DC. Chloride secretion by semicircular canal duct epithelium is stimulated via β2-adrenergic receptors. Am J Physiol, Cell Physiol. 2002;283:C1752–C1760. - PubMed
-
- Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green E, Wall SM, Marcus DC. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol. 2007;292:F1314–F1321. doi: 10.1152/ajprenal.00432.2006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

