Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment
- PMID: 20398273
- PMCID: PMC2888222
- DOI: 10.1186/ar2984
Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment
Abstract
Introduction: Patients with rheumatoid arthritis (RA) have an increased risk of infection and this risk appears to be higher with anti-TNF (tumor necrosis factor) agents. We pooled data from the cumulative abatacept RA clinical development program, both double-blind and open-label periods, to estimate the incidence rates (IRs) of infections requiring hospitalization including pneumonia and opportunistic infections, in comparison with RA patients treated with non-biologic disease-modifying antirheumatic drugs (DMARDs) from several reference cohorts.
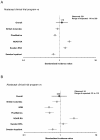
Methods: Infections reported in seven abatacept clinical trials of RA patients (double-blind and open-label periods) were tabulated. Comparisons were made between the observed IRs in abatacept-treated patients and those in over 133,000 patients exposed to non-biologic DMARDs in six reference RA cohorts. Age- and sex-adjusted IRs of infections requiring hospitalization, including pneumonia (most frequent hospital infection), were used to estimate the expected IRs with abatacept by the method of indirect adjustment. Standardized incidence ratios (SIR) and 95% CI were calculated comparing incidence in the cumulative abatacept experience with incidence in each RA cohort.
Results: A total of 1,955 (double-blind period) and 4,134 (double-blind + open-label periods with a cumulative exposure of 8,392 person-years) abatacept-treated RA patients were analyzed. Observed IRs for infections requiring hospitalization during the double-blind period were 3.05 per 100-patient years for abatacept-treated patients and 2.15 per 100 patient years for placebo. In the cumulative population, observed IR for infections requiring hospitalization was 2.72 per 100-patient years. Rates for abatacept were similar to expected IRs based on other RA non-biologic DMARD cohorts.
Conclusions: IRs of infections requiring hospitalization and pneumonia in abatacept trials are consistent with expected IRs based on reference RA DMARD cohorts. RA patients are at higher risk of infection compared with the general population, making the RA DMARD cohorts an appropriate reference group. The safety of abatacept, including incidence of infections requiring hospitalization, will continue to be monitored in a post-marketing surveillance program.
Figures
References
-
- Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, Chan KA. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35:387–393. - PubMed
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. - DOI - PubMed
-
- Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896–2904. doi: 10.1002/art.22808. - DOI - PMC - PubMed
-
- Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

