Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial
- PMID: 20398352
- PMCID: PMC2879572
- DOI: 10.1186/bcr2565
Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial
Abstract
Introduction: The aim of this multicenter, phase III, prospective open label clinical trial was to investigate the effect of risedronate (R) on bone mineral density (BMD) in postmenopausal, early breast cancer (BC) patients scheduled to receive anastrozole (A).
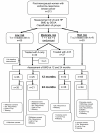
Methods: Pre-treatment BMD of 213 patients with hormone receptor-positive BC was evaluated at lumbar spine (LS) and hip (HP). Patients were categorized according to their baseline BMD T-score as being at low, moderate and high risk of osteoporosis. Low risk patients received anastrozole only (A), moderate risk were randomized to anastrozole +/- risedronate (A+/-R) administration and high risk patients received anastrozole + risedronate (A+R). Anastrozole was given at a dosage of 1 mg/day while oral risedronate was given at 35 mg/week. BMD was then assessed at 12 and 24 months. All patients received daily supplements of calcium (1000 mg/day) and vitamin D (400 IU/day).
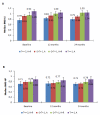
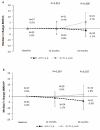
Results: At 24 months, in the moderate risk group, treatment with A+R resulted in a significant increase in BMD at LS and HP compared to treatment with A only (5.7% v -1.5%, Wilcoxon test P = 0.006, and 1.6% v -3.9% Wilcoxon test P = 0.037, respectively), while no significant difference was found at 12 months; 24.3% of the patients moved to normal BMD region. In the high risk group, a significant increase for LS was detected both at 12 and 24 months (6.3% and 6.6%, P < 0.001) but not for HP; BMD in 14% of patients improved to the osteopenic region. In the low risk group, a significant decrease of BMD was detected at 12 months for LS and HP (-5.3% P < 0.001 and -2.4% P < 0.001, respectively,); at 24 months, a significant decrease of BMD was detected only for LS (-2.5%, P < 0.001). However, 22% of patients became osteopenic and only 4% became osteoporotic.
Conclusions: The addition of oral risedronate in post-menopausal breast cancer patients receiving anastrozole has a favorable effect on BMD. Patients with pre-treatment osteopenic to osteoporotic status should be treated with a combination of both therapies in order to avoid bone loss induced by aromatase inhibition. Patients with normal BMD before starting treatment with anastrozole have a very low risk to develop osteoporosis.
Trial registration: ClinicalTrials.gov NCT00809484.
Figures
Comment in
-
Managing bone mineral density with oral bisphosphonate therapy in women with breast cancer receiving adjuvant aromatase inhibition.Breast Cancer Res. 2010;12(3):110. doi: 10.1186/bcr2584. Epub 2010 Jun 18. Breast Cancer Res. 2010. PMID: 20584345 Free PMC article.
References
-
- Raisz LG. In: Osteoporosis. Marcus R, Feldman D, Kesley J, editor. New York: Academic Press; 1966. Interaction of local and systemic factors of the pathogenesis of osteoporosis.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

