Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure
- PMID: 20398635
- PMCID: PMC4452019
- DOI: 10.1016/j.brainres.2010.04.013
Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure
Abstract
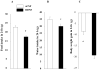
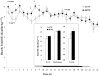
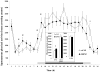
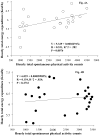
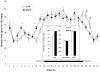
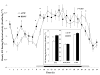
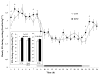
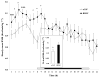
Brain-derived neurotrophic factor (BDNF) decreases food intake and body weight, but few central sites of action have been identified for its effect on energy expenditure. The hypothalamic ventromedial nucleus (VMH) is important in regulating energy metabolism. Our previous work indicated that BDNF in the VMH reduced food intake. The purposes of the study were to determine: 1) if BDNF in the VMH increases energy expenditure (EE); 2) if BDNF-enhanced thermogenesis results from increased spontaneous physical activity (SPA) and resting metabolic rate (RMR); and 3) if VMH BDNF thermogenic effects are mediated by uncoupling protein 1 (UCP1) in brown adipose tissue (BAT). BDNF (0.5 microg) was injected into the VMH of male Sprague-Dawley rats and oxygen consumption, carbon dioxide production, food intake and SPA were measured for 24h in an indirect calorimeter. Animals were sacrificed 4h after BDNF injection, and BAT UCP1 gene expression was measured with quantitative real-time polymerase chain reaction. BDNF significantly decreased food and water intake, and body weight gain. Heat production and RMR were significantly elevated for 9h immediately after BDNF injection. BDNF increased SPA and EE during SPA (aEE) within 9h after injection although BDNF had no effect on 0-24h SPA and aEE. BDNF did not induce a significant increase in BAT UCP1 expression. In conclusion, VMH BDNF reduces body weight by decreasing food intake and increasing EE consequent to increased SPA and RMR, suggesting that the VMH is an important site of BDNF action to influence energy balance.
Published by Elsevier B.V.
Figures
Similar articles
-
Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate.Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R992-1002. doi: 10.1152/ajpregu.00516.2006. Epub 2007 Jun 13. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17567712
-
Fasting increases gene expressions of uncoupling proteins and peroxisome proliferator-activated receptor-gamma in brown adipose tissue of ventromedial hypothalamus-lesioned rats.Life Sci. 2003 May 16;72(26):3035-46. doi: 10.1016/s0024-3205(03)00225-x. Life Sci. 2003. PMID: 12706490
-
Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake.Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1037-45. doi: 10.1152/ajpregu.00125.2007. Epub 2007 Jun 6. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17553842
-
Negative regulators of brown adipose tissue (BAT)-mediated thermogenesis.J Cell Physiol. 2014 Dec;229(12):1901-7. doi: 10.1002/jcp.24664. J Cell Physiol. 2014. PMID: 24809334 Free PMC article. Review.
-
The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight.Physiol Behav. 2006 Feb 28;87(2):221-44. doi: 10.1016/j.physbeh.2005.10.007. Epub 2006 Jan 18. Physiol Behav. 2006. PMID: 16412483 Review.
Cited by
-
Brown adipose tissue: development, metabolism and beyond.Biochem J. 2013 Jul 15;453(2):167-78. doi: 10.1042/BJ20130457. Biochem J. 2013. PMID: 23805974 Free PMC article. Review.
-
Anti-Seizure and Neuronal Protective Effects of Irisin in Kainic Acid-Induced Chronic Epilepsy Model with Spontaneous Seizures.Neurosci Bull. 2022 Nov;38(11):1347-1364. doi: 10.1007/s12264-022-00914-w. Epub 2022 Jul 12. Neurosci Bull. 2022. PMID: 35821335 Free PMC article.
-
Vagus nerve stimulation reduces body weight and fat mass in rats.PLoS One. 2012;7(9):e44813. doi: 10.1371/journal.pone.0044813. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23028630 Free PMC article.
-
MANF: A New Player in the Control of Energy Homeostasis, and Beyond.Front Physiol. 2018 Nov 29;9:1725. doi: 10.3389/fphys.2018.01725. eCollection 2018. Front Physiol. 2018. PMID: 30555354 Free PMC article. Review.
-
Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure.Mol Psychiatry. 2022 Jun;27(6):2901-2913. doi: 10.1038/s41380-022-01513-x. Epub 2022 Mar 22. Mol Psychiatry. 2022. PMID: 35318460 Free PMC article.
References
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–78. - PubMed
-
- Bannai M, Ichikawa M, Nishihara M, Takahashi M. Effect of injection of antisense oligodeoxynucleotides of GAD isozymes into rat ventromedial hypothalamus on food intake and locomotor activity. Brain Res. 1998;784:305–15. - PubMed
-
- Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–20. - PubMed
-
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB. Effects of NPY and NPY2-36 on body temperature and food intake following administration into hypothalamic nuclei. Brain Res Bull. 1995;36:131–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

