Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation
- PMID: 20398648
- PMCID: PMC3098388
- DOI: 10.1016/j.ydbio.2010.04.005
Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation
Abstract
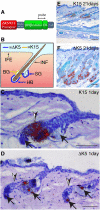
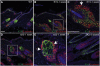
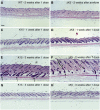
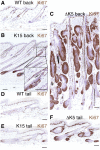
Wnt signalling is required for hair follicle development and for the growth phase (anagen) of postnatal follicles. When the pathway is activated at high levels in adult mouse epidermis, ectopic follicles form from existing follicles, interfollicular epidermis (IFE) and sebaceous glands, revealing a remarkable ability of the tissue to be reprogrammed. To compare the competence of different epidermal cell populations to form ectopic follicles, we expressed a 4-hydroxy-tamoxifen (4OHT) inducible, stabilised beta-catenin transgene (DeltaNbeta-cateninER) under the control of two different promoters. We targeted the reservoir of stem cells in the hair follicle bulge via the keratin 15 (K15) promoter and targeted the sebaceous glands and base of the follicle (bulb) with a truncated K5 promoter (DeltaK5). No ectopic follicles formed in the IFE in either model, establishing the autonomy of the IFE stem cell compartment in undamaged epidermis. Activation of beta-catenin in the bulge stimulated proliferation and bulge expansion. Existing hair follicles entered anagen, but no ectopic follicles formed. DeltaK5DeltaNbeta-cateninER expressing hair follicles also entered anagen on 4OHT treatment. In addition, a subpopulation of cells at the base of the sebaceous gland readily formed ectopic follicles, resulting in complete and reversible conversion of sebaceous glands into hair follicles. Combined activation of beta-catenin and the vitamin D receptor enhanced differentiation of sebaceous gland-derived hair follicles and stimulated ectopic follicle formation in the hair follicle bulb, but not in the bulge. Our results suggest that the bulge and sebaceous gland are, respectively, non-permissive and permissive niches for Wnt induced hair follicle differentiation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




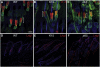
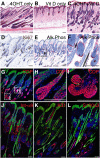
References
-
- Andl T., Reddy S.T., Gaddapara T., Millar S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002;2:643–653. - PubMed
-
- Ballester M., Castelló A., Ibáñez E., Sánchez A., Folch J.M. Real-time quantitative PCR-based system for determining transgene copy number in transgenic animals. BioTechniques. 2004;37:610–613. - PubMed
-
- Braun K.M., Niemann C., Jensen U.B., Sundberg J.P., Silva-Vargas V., Watt F.M. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. - PubMed
-
- Brown K., Strathdee D., Bryson S., Lambie W., Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol. 1998;8:516–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

