Structural and functional analysis of the hemagglutinin-esterase of infectious salmon anaemia virus
- PMID: 20398710
- PMCID: PMC7114507
- DOI: 10.1016/j.virusres.2010.03.020
Structural and functional analysis of the hemagglutinin-esterase of infectious salmon anaemia virus
Abstract
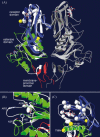
Infectious salmon anaemia virus (ISAV) is a piscine orthomyxovirus causing a serious disease in farmed Atlantic salmon (Salmo salar L.). The virus surface glycoprotein hemagglutinin-esterase (HE) is responsible for both viral attachment and release. Similarity to bovine and porcine torovirus hemagglutinin-esterase (BToV HE, PToV HE), bovine coronavirus HE (BCoV HE) and influenza C hemagglutinin-esterase-fusion (InfC HEF) proteins were exploited in a computational homology-based structure analysis of ISAV HE. The analysis resolved structural aspects of the protein and identified important features of relevance to ISAV HE activity. By recombinant expression and purification of secretory HE (recHE) proteins, receptor-binding and quantitative analyses of enzymatic activities displayed by ISAV HE molecules are presented for the first time. Three different recHE molecules were constructed: one representing a high virulent isolate, one a low virulent, while in the third a Ser(32) to Ala(32) amino acid substitution was introduced in the enzymatic catalytic site as inferred from the model. The three amino acid differences between the high and low virulent variants, of which two localized to the putative receptor-binding domain and one in the esterase domain, had no impact on receptor-binding or -release activities. In contrast, the Ser(32) amino acid substitution totally abolished enzymatic activity while receptor binding increased, as observed by agglutination of Atlantic salmon red blood cells. This demonstrates the essential role of a serine in the enzyme's catalytic site. In conclusion, structural analysis of ISAV HE in combination with selected recHE proteins gave insights into structure-function relationships and opens up for further studies aiming at dissecting molecular determinants of ISAV virulence.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Structure of the infectious salmon anemia virus receptor complex illustrates a unique binding strategy for attachment.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):E2929-E2936. doi: 10.1073/pnas.1617993114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320973 Free PMC article.
-
Expression, antigenicity and studies on cell receptor binding of the hemagglutinin of infectious salmon anemia virus.Arch Virol. 2005 Aug;150(8):1621-37. doi: 10.1007/s00705-005-0502-4. Epub 2005 Apr 14. Arch Virol. 2005. PMID: 15824888
-
Evolutionary mechanisms involved in the virulence of infectious salmon anaemia virus (ISAV), a piscine orthomyxovirus.Virology. 2008 May 10;374(2):515-27. doi: 10.1016/j.virol.2008.01.019. Epub 2008 Feb 20. Virology. 2008. PMID: 18280528
-
SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction.Int J Mol Sci. 2020 Jun 26;21(12):4549. doi: 10.3390/ijms21124549. Int J Mol Sci. 2020. PMID: 32604730 Free PMC article. Review.
-
Infectious salmon anemia virus: causative agent, pathogenesis and immunity.Anim Health Res Rev. 2004 Jun;5(1):65-78. doi: 10.1079/ahr200461. Anim Health Res Rev. 2004. PMID: 15460541 Review.
Cited by
-
Identification and localization of the structural proteins of anguillid herpesvirus 1.Vet Res. 2011 Oct 5;42(1):105. doi: 10.1186/1297-9716-42-105. Vet Res. 2011. PMID: 21975111 Free PMC article.
-
Chemical Synthesis and In Vitro Evaluation of a Phage Display-Derived Peptide Active against Infectious Salmon Anemia Virus.Appl Environ Microbiol. 2016 Apr 4;82(8):2563-2571. doi: 10.1128/AEM.00184-16. Print 2016 Apr. Appl Environ Microbiol. 2016. PMID: 26896129 Free PMC article.
-
Structure of the infectious salmon anemia virus receptor complex illustrates a unique binding strategy for attachment.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):E2929-E2936. doi: 10.1073/pnas.1617993114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320973 Free PMC article.
-
Interaction of the Amino-Terminal Domain of the ISAV Fusion Protein with a Cognate Cell Receptor.Pathogens. 2020 May 27;9(6):416. doi: 10.3390/pathogens9060416. Pathogens. 2020. PMID: 32471165 Free PMC article.
-
Destruction of the vascular viral receptor in infectious salmon anaemia provides in vivo evidence of homologous attachment interference.PLoS Pathog. 2022 Oct 14;18(10):e1010905. doi: 10.1371/journal.ppat.1010905. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36240255 Free PMC article.
References
-
- Anonymous Epizootiological investigation into a case of suspicion of infectious salmon anaemia (ISA) in Scotland in November 2004. Report by FRS Marine Laboratory; Aberdeen; 2005.
-
- Baigent S.J., McCauley J.W. Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res. 2001;79(1–2):177–185. - PubMed
-
- Barton G.J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993;6(1):37–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

