Delivery of paclitaxel from cobalt-chromium alloy surfaces without polymeric carriers
- PMID: 20398928
- PMCID: PMC4076912
- DOI: 10.1016/j.biomaterials.2010.03.043
Delivery of paclitaxel from cobalt-chromium alloy surfaces without polymeric carriers
Abstract
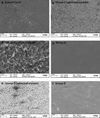
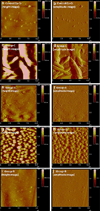
Polymer-based carriers are commonly used to deliver drugs from stents. However, adverse responses to polymer coatings have raised serious concerns. This research is focused on delivering drugs from stents without using polymers or any carriers. Paclitaxel (PAT), an anti-restenotic drug, has strong adhesion towards a variety of material surfaces. In this study, we have utilized such natural adhesion property of PAT to attach these molecules directly to cobalt-chromium (Co-Cr) alloy, an ultra-thin stent strut material. Four different groups of drug coated specimens were prepared by directly adding PAT to Co-Cr alloy surfaces: Group-A (PAT coated, unheated, and ethanol cleaned); Group-B (PAT coated, heat treated, and ethanol cleaned); Group-C (PAT coated, unheated, and not ethanol cleaned); and Group-D (PAT coated, heat treated and not ethanol cleaned). In vitro drug release of these specimens was investigated using high performance liquid chromatography. Groups A and B showed sustained PAT release for up to 56 days. A simple ethanol cleaning procedure after PAT deposition can remove the loosely bound drug crystals from the alloy surfaces and thereby allowing the remaining strongly bound drug molecules to be released at a sustained rate. The heat treatment after PAT coating further improved the stability of PAT on Co-Cr alloy and allowed the drug to be delivered at a much slower rate, especially during the initial 7 days. The specimens which were not cleaned in ethanol, Groups C and D, showed burst release. PAT coated Co-Cr alloy specimens were thoroughly characterized using scanning electron microscopy, atomic force microscopy, and X-ray photoelectron spectroscopy. These techniques were collectively useful in studying the morphology, distribution, and attachment of PAT molecules on Co-Cr alloy surfaces. Thus, this study suggests the potential for delivering paclitaxel from Co-Cr alloy surfaces without using any carriers.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures








Similar articles
-
Paclitaxel delivery from cobalt-chromium alloy surfaces using self-assembled monolayers.Biointerphases. 2011 Jun;6(2):33-42. doi: 10.1116/1.3575530. Biointerphases. 2011. PMID: 21721838
-
Microrough cobalt-chromium alloy surfaces for paclitaxel delivery: preparation, characterization, and in vitro drug release studies.Langmuir. 2012 Aug 7;28(31):11511-26. doi: 10.1021/la301636z. Epub 2012 Jul 26. Langmuir. 2012. PMID: 22720656
-
Vitamin-C delivery from CoCr alloy surfaces using polymer-free and polymer-based platforms for cardiovascular stent applications.Langmuir. 2014 Jun 3;30(21):6237-49. doi: 10.1021/la501448h. Epub 2014 May 21. Langmuir. 2014. PMID: 24832897
-
Interaction of endothelial and smooth muscle cells with cobalt-chromium alloy surfaces coated with paclitaxel deposited self-assembled monolayers.Langmuir. 2013 Nov 19;29(46):14254-64. doi: 10.1021/la403533r. Epub 2013 Nov 6. Langmuir. 2013. PMID: 24156365
-
Long-term stability of self-assembled monolayers on electropolished L605 cobalt chromium alloy for stent applications.J Biomed Mater Res B Appl Biomater. 2011 Aug;98(2):280-9. doi: 10.1002/jbm.b.31850. Epub 2011 May 20. J Biomed Mater Res B Appl Biomater. 2011. PMID: 21604365
Cited by
-
Macromolecular approaches to prevent thrombosis and intimal hyperplasia following percutaneous coronary intervention.Biomacromolecules. 2014 Aug 11;15(8):2825-32. doi: 10.1021/bm5007757. Epub 2014 Jul 8. Biomacromolecules. 2014. PMID: 24964369 Free PMC article. Review.
References
-
- Grundy SM. Atlas of atherosclerosis: risk factors and treatment. 4th ed. Philadelphia: Current Medicine Group; 2005.
-
- Serruys PW, Jaegere PD, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331(8):489–495. - PubMed
-
- Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331(8):496–501. - PubMed
-
- Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44(4):733–739. - PubMed
-
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

